近日,郑州大学张开翔团队报道了跨膜DNA纳米通道工程人工受体导航NK细胞免疫治疗实体瘤。这一研究成果发表在2026年2月4日出版的《美国化学会志》上。
针对实体瘤的过继性自然杀伤(NK)细胞疗法面临关键挑战,包括肿瘤抗原异质性、肿瘤浸润能力受限以及免疫抑制微环境导致的细胞活化不足。
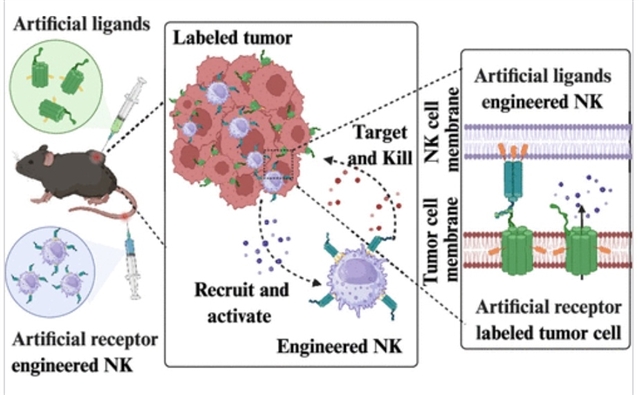
研究组开发了一种工程化纳米平台,其核心特征为跨膜DNA纳米通道工程化人工受体,通过双重协同机制引导NK细胞靶向实体瘤:1)肿瘤微环境重编程:利用胆固醇介导的膜插入作用,NCAR可整合至肿瘤细胞膜并破坏磷脂双分子层,诱导免疫原性细胞死亡并释放损伤相关分子模式(如HMGB1、CRT),从而重塑免疫抑制性肿瘤微环境并招募/激活NK细胞;2)精准靶向:NCAR通过与NK细胞表面设计的DNA纳米人工配体碱基配对,形成可编程合成免疫突触。
这种不依赖抗原的组装网络建立了通用膜界面,可实现持续肿瘤靶向的NK细胞活化。该双组件系统使NK细胞在瘤内持续积聚超96小时,活化的NKP46+GZB+ NK细胞数量较对照组提升15.1倍。通过将DNA纳米技术与细胞免疫疗法相结合,该纳米平台为调控肿瘤-免疫相互作用提供了通用策略,有望突破过继性NK细胞疗法在实体瘤治疗中的关键局限。
附:英文原文
Title: Transmembrane DNA Nanochannel-Engineered Artificial Receptors for Navigating NK Cell Immunotherapy in Solid Tumors
Author: Danyu Wang, Hua Yi, Jiali Zhang, Mengyu Huang, Yue Qiu, Yang Wang, Peiru Chen, Chan Liu, Tingyi Xu, Qiuxia Yang, Kuikun Yang, Zhenzhen Guo, Kaixiang Zhang
Issue&Volume: February 4, 2026
Abstract: Adoptive natural killer (NK) cell therapy for solid tumors faces critical challenges, including tumor antigen heterogeneity, impaired tumor infiltration, and suboptimal activation imposed by the immunosuppressive microenvironment. Here we developed an engineered nanoplatform featuring transmembrane DNA nanochannel-engineered artificial receptors (NCAR) to direct NK cells against solid tumors through two synergistic mechanisms: 1) Tumor Microenvironment (TME) Reprogramming: leveraging cholesterol-mediated insertion, NCAR incorporates into tumor membranes to disrupt phospholipid bilayers, inducing immunogenic cell death with the release of damage-associated molecular patterns (DAMPs; e.g., HMGB1, CRT), which remodels immunosuppression TME and recruits/activates NK cells. 2) Precision Targeting: NCAR forms programmable synthetic immune synapses with DNA nanoartificial ligands (NAL) engineered on NK cells via base-pairing. This antigen-independent assembly network establishes a universal membrane interface, enabling sustained tumor-targeted NK cell activation. The dual-component system enables sustained intratumoral accumulation of NK cells (>96 h), with a 15.1-fold increase in activated NKP46+GZB+ NK cells compared to controls. By bridging DNA nanotechnology with cell immunotherapy, our nanoplatform provides a universal strategy for navigating tumor-immune interactions, addressing key limitations of adoptive NK cell immunotherapy in solid tumors.
DOI: 10.1021/jacs.5c16425
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c16425
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
