近日,武汉大学孔望清团队研究了各种C(sp3) -H键与醛的直接和对映选择性酰化。相关论文于2026年2月12日发表在《美国化学会志》上。
含有α-芳基、α-氨基或α-氧立体中心的手性酮是众多重要天然产物和药物中的独特结构单元,但其对映选择性合成仍具挑战。
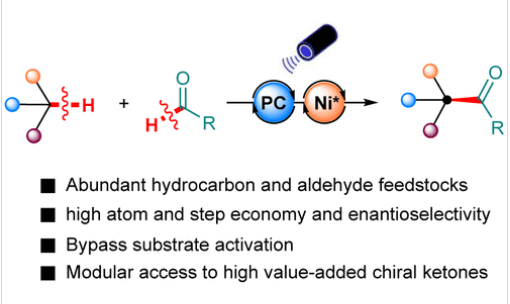
研究组报道了一种通过过氧化物光敏化与镍催化协同作用,实现多样化C(sp3)-H键对映选择性酰基化的合成方法。该方法以来源广泛、廉价易得的醛类为酰基源,可对苄位、α-氨基及α-烷氧基等多种C(sp3)-H键进行酰基化,兼具优异对映选择性与原子经济性。该策略在手性合成砌块及具有生物活性的天然产物、药物对映选择性合成中的实用性已得到验证。
这一方法为自由基-自由基直接交叉偶联反应中立体选择性控制的难题提供了前所未有的解决方案。机理研究表明,可见光诱导的三重态能量转移可促进过氧化物在温和条件下分解生成烷氧自由基,该自由基作为氢原子转移试剂分别生成酰基自由基与底物自由基,而手性镍催化则负责这两个自由基的不对称交叉偶联,最终形成C(sp3)-H酰基化产物。
附:英文原文
Title: Direct and Enantioselective Acylation of Diverse C(sp3)–H Bonds with Aldehydes
Author: Zhijun Zhou, Fen Hu, Xinjing Lin, Yuanyuan Ping, Wangqing Kong
Issue&Volume: February 12, 2026
Abstract: Chiral ketones containing α-aryl, α-amino, or α-oxy stereocenters are unique structural motifs found in numerous important natural products and pharmaceuticals, but their enantioselective synthesis remains a challenge. We report a synthetic method for the enantioselective acylation of diverse C(sp3)–H bonds by combining peroxide photosensitization and nickel catalysis. This method utilizes abundant and readily available aldehydes as an acyl source and is capable of acylating a variety of C(sp3)–H bonds, including benzylic, α-amino, and α-alkoxy C(sp3)–H bonds, with excellent enantioselectivity and atom economy. The practicability of this strategy is demonstrated in the enantioselective synthesis of chiral building blocks as well as biologically active natural products and pharmaceuticals. This method provides an unprecedented solution to the challenging problem of stereoselective control in various radical–radical direct cross-coupling reactions. Mechanistic studies revealed that visible light triplet energy transfer promotes the mild decomposition of peroxides to generate alkoxy radicals, which act as hydrogen atom transfer reagents to generate acyl radicals and substrate radicals, respectively, while chiral nickel catalysis is responsible for the asymmetric cross-coupling of these two radicals to generate C(sp3)–H acylated products.
DOI: 10.1021/jacs.5c18382
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c18382
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
