近日,中国科学院上海有机所施世良团队报道了反电负性金属转化使烯烃碳镁化反应进入季碳中心。相关论文于2026年2月11日发表在《自然-化学》杂志上。
格氏试剂作为合成化学的基石,其应用长期受限于复杂骨架的构筑难题,这已成为持续存在的合成瓶颈。与此同时,广泛存在于生物活性分子与天然产物中的季碳立体中心,虽经数十年研究仍属艰巨的合成目标。
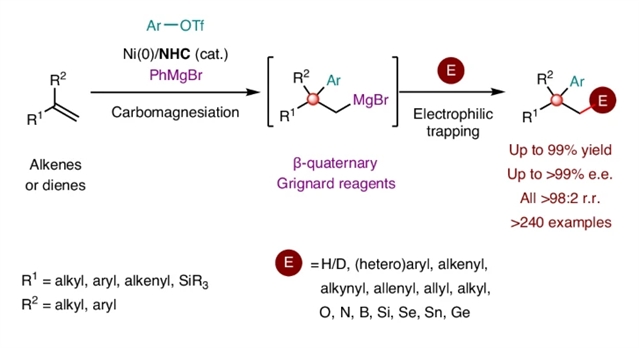
研究组报道了一种镍催化碳镁化策略,通过罕见的反电负性金属交换(镍至镁)同步攻克上述挑战。该策略以芳基三氟甲磺酸酯与PhMgBr分别作为碳源和镁源,通过1,1-二取代烯烃及1,3-二烯的碳镁化反应,实现了β-季碳格氏试剂的高效模块化合成。
所得有机镁试剂可与多种亲电试剂发生一锅反应,高精度构筑立体化学复杂的季碳中心。机理研究表明,基于大位阻N-杂环卡宾的催化剂颠覆了经典交叉偶联路径,迫使反直觉的镍至镁金属交换发生——这一过程既违背传统电负性规律,又实现了卓越的区域与对映选择性控制。该反电负性金属交换策略展现出推动碳金属化反应发展的巨大潜力,并为交叉偶联化学开辟了新航道。
附:英文原文
Title: Contra-electronegativity transmetallation unlocks alkene carbomagnesiation to access quaternary stereocentres
Author: Ye, Xiaodong, Sun, Bo, Shi, Shi-Liang
Issue&Volume: 2026-02-11
Abstract: Grignard reagents—cornerstones of synthetic chemistry—are hindered by enduring limitations in accessing complex architectures, which poses a persistent synthetic bottleneck. Meanwhile, quaternary carbon (stereo)centres, ubiquitous in bioactive molecules and natural products, remain formidable synthetic targets despite decades of research. Here we introduce a nickel-catalysed carbomagnesiation strategy that simultaneously overcomes these challenges through a rare contra-electronegativity transmetallation (Ni to Mg). This approach enables the efficient and modular synthesis of β-quaternary Grignard reagents via carbomagnesiation of 1,1-disubstituted alkenes and 1,3-dienes, employing aryl triflate and PhMgBr as carbon and magnesium sources, respectively. The resulting organomagnesium reagents undergo one-pot reactions with diverse electrophiles, delivering stereochemically complex quaternary centres with high precision. Mechanistically, bulky N-heterocyclic carbene (NHC)-based catalysts divert classical cross-coupling pathways, enforcing a counterintuitive Ni-to-Mg transmetallation that defies conventional electronegativity trends while achieving exceptional regio- and enantiocontrol. This contra-electronegativity transmetallation demonstrates substantial potential to advance carbometallation reactions and open new avenues for cross-coupling chemistry.
DOI: 10.1038/s41557-026-02073-1
Source: https://www.nature.com/articles/s41557-026-02073-1
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
