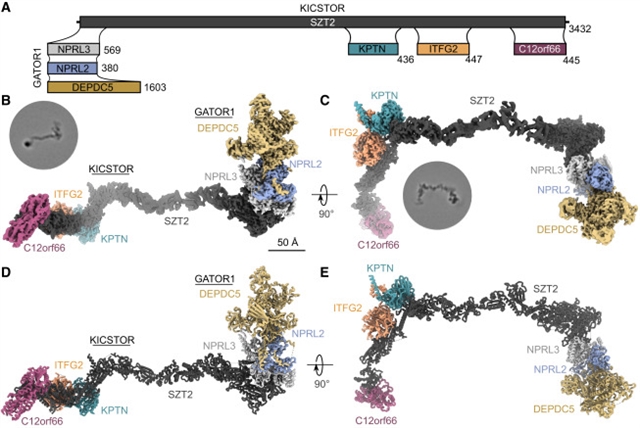
莫纳什大学Andrew M. Ellisdon团队取得一项新突破。他们揭示了溶酶体KICSTOR-GATOR1-SAMTOR营养感应超复合体的结构。相关论文于2026年1月8日发表在《细胞》杂志上。
该课题组人员解析了KICSTOR-GATOR1结构,揭示了一个引人注目的~60纳米月牙形组装。GATOR1通过广泛的接口锚定在KICSTOR上,破坏这种相互作用的突变会损害mTORC1的调节。S-腺苷蛋氨酸传感器SAMTOR以与代谢物结合不相容的方式与KICSTOR结合,通过SAMTOR-KICSTOR结合提供了对蛋氨酸传感的结构见解。研究小组发现KICSTOR和GATOR1形成一个二聚体超配合物。该组装将GATOR1限制在有利于Rag GTPase结合的低亲和力活性GAP模式的取向上,而在空间上限制了进入高亲和力抑制模式的途径,这与活性溶酶体GATOR1对接复合物的模型一致。
据介绍,异二聚体Rag gtpase的鸟苷三磷酸(GTP)结合状态作为调节氨基酸波动下游溶酶体中雷帕霉素复合物1 (mTORC1)激活的机制靶点的分子开关。在低氨基酸条件下, GTPase激活蛋白(GAP)对Rags 1 (GATOR1)的活性促进RagA GTP水解,阻止mTORC1的激活。KICSTOR通过未定义的机制在溶酶体上招募和调节GATOR1。
附:英文原文
Title: Structure of the lysosomal KICSTOR-GATOR1-SAMTOR nutrient-sensing supercomplex
Author: Christopher J. Lupton, Charles Bayly-Jones, Shuqi Dong, Terrance Lam, Wentong Luo, Gareth D. Jones, Chantel Mastos, Nicholas J. Frescher, San S. Lim, Alastair C. Keen, Luke E. Formosa, Hari Venugopal, Yong-Gang Chang, Michelle L. Halls, Andrew M. Ellisdon
Issue&Volume: 2026-01-08
Abstract: The guanosine triphosphate (GTP)-bound state of the heterodimeric Rag GTPases functions as a molecular switch regulating mechanistic target of rapamycin complex 1 (mTORC1) activation at the lysosome downstream of amino acid fluctuations. Under low amino acid conditions, GTPase-activating protein (GAP) activity toward Rags 1 (GATOR1) promotes RagA GTP hydrolysis, preventing mTORC1 activation. KICSTOR recruits and regulates GATOR1 at the lysosome by undefined mechanisms. Here, we resolve the KICSTOR-GATOR1 structure, revealing a striking ~60-nm crescent-shaped assembly. GATOR1 anchors to KICSTOR via an extensive interface, and mutations that disrupt this interaction impair mTORC1 regulation. The S-adenosylmethionine sensor SAMTOR binds KICSTOR in a manner incompatible with metabolite binding, providing structural insight into methionine sensing via SAMTOR-KICSTOR association. We discover that KICSTOR and GATOR1 form a dimeric supercomplex. This assembly restricts GATOR1 to an orientation that favors the low-affinity active GAP mode of Rag GTPase engagement while sterically restricting access to the high-affinity inhibitory mode, consistent with a model of an active lysosomal GATOR1 docking complex.
DOI: 10.1016/j.cell.2025.12.005
Source: https://www.cell.com/cell/abstract/S0092-8674(25)01418-7
