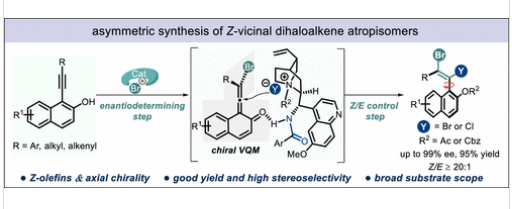
近日,重庆大学闫海龙团队报道了炔烃立体选择性双卤化制备手性Z型邻二卤烯烃阻转异构体。相关论文于2025年12月30日发表在《美国化学会志》上。
Z构型烯烃的立体选择性构建仍是合成化学中的一个根本性挑战,尤其是在需要同时引入具有高手性选择性的其他立体生成单元时。此外,邻二卤烯烃是有机化学和药物化学中的优势骨架,而制备邻二卤烯烃的传统方法依赖于卤鎓离子中间体介导的途径,且通常生成E构型产物。
研究组报道了一种催化立体选择性合成高对映体富集的Z构型邻二卤烯烃阻转异构体的方法,该方法以高产率(高达95%)、优异的对映选择性(高达99%)和超过20:1的Z/E选择性实现转化。该转化是通过以易于获得的N-溴代丁二酰亚胺(NBS)和酰卤作为卤化试剂,经丙二烯介导的有机催化组装过程实现的。
附:英文原文
Title: Stereoselective Dihalogenation of Alkynes to Access Enantioenriched Z-Vicinal Dihaloalkene Atropisomers
Author: Yu Chang, Da Xu, Guojie Zhou, Bangli Liu, Haitao Zhou, Wenling Qin, Hailong Yan
Issue&Volume: December 30, 2025
Abstract: The stereoselective construction of Z-configured olefins remains a fundamental challenge in synthetic chemistry, particularly when additional stereogenic elements with high enantioselectivity are simultaneously introduced. Furthermore, vicinal dihaloalkenes are privileged scaffolds in organic and medicinal chemistry, and the traditional protocol for preparing vicinal dihaloalkenes relies on a halonium intermediate–mediated pathway and typically yields E-configured adducts. Herein, we report the catalytic stereoselective synthesis of enantioenriched Z-vicinal dihaloalkene atropisomers in high yields (up to 95%) with excellent enantioselectivity (up to 99%) and a Z/E ratio of more than 20:1. This transformation was achieved through an allene-mediated organocatalyzed assembly of readily available N-Bromosuccinimide (NBS) and acyl halides as halogenation reagents.
DOI: 10.1021/jacs.5c14722
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c14722
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
