近日,美国加州大学洛杉矶分校Garg, Neil K.团队研究了键级接近1.5的超锥体化烯烃可作为合成构件。2026年1月21日出版的《自然-化学》杂志发表了这项成果。
烯烃分子通常在两端呈现三角平面几何构型,其稳定的σ键与π键共同形成约2的键级。
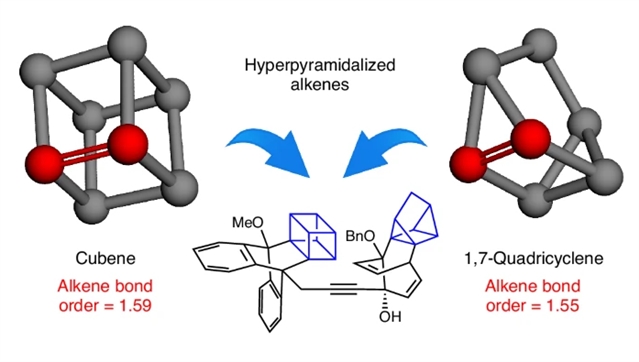
研究组聚焦于具有极端几何形变特征的特殊烯烃——这类烯烃呈现高度超锥形化结构。在超锥形化烯烃中,分子几何构型显著偏离典型的三角平面构型,导致π键作用减弱,烯烃键级异常趋近于1.5。立方烯与1,7-四环烷分别于1988年和1979年被首次证实存在,但此后数十年未受关注,现成为本研究重点。研究组利用其异常脆弱的π键开展环加成反应,成功构建复杂分子骨架并拓展了前所未有的化学空间。通过计算化学方法深入探究了这种异常低键级的成因。这些研究预计将推动未来对超锥形化分子及非典型键级体系的探索。
附:英文原文
Title: Hyperpyramidalized alkenes with bond orders near 1.5 as synthetic building blocks
Author: Ding, Jiaming, French, Sarah A., Rivera, Christina A., Tena Meza, Arismel, Witkowski, Dominick C., Houk, K. N., Garg, Neil K.
Issue&Volume: 2026-01-21
Abstract: Alkenes typically have trigonal planar geometries at each terminus, with favourable σ- and π-bonding leading to a bond order of ~2. Here we consider unusual alkenes that possess an extreme form of geometric distortion, termed hyperpyramidalization. In a hyperpyramidalized alkene, geometries deviate remarkably from the typical trigonal planar alkene geometry, leading to weak π-bonding and abnormal alkene bond orders approaching 1.5. Cubene and 1,7-quadricyclene, first validated in 1988 and 1979, respectively, but overlooked for decades since, are the focus of the present study. We leverage their unusually weak π-bonds in cycloadditions, enabling the construction of complex scaffolds and access to previously unrealized chemical space. The origins of the unusually low bond orders were investigated using computational methods. These efforts are expected to prompt future studies of molecules that display hyperpyramidalization or atypical bond orders.
DOI: 10.1038/s41557-025-02055-9
Source: https://www.nature.com/articles/s41557-025-02055-9
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
