近日,英国牛津大学Steel, Harrison团队研究了多模态传感工程蛋白中的量子自旋共振。相关论文于2026年1月21日发表在《自然》杂志上。
利用量子现象进行测量的传感技术,正日益广泛地应用于材料、物理和生物科学领域。直到最近,量子传感器的生物候选对象仍局限于体外系统,且存在灵敏度低、易受光致降解等问题。这些限制阻碍了其实际生物技术应用以及有助于其工程化与优化的高通量研究。
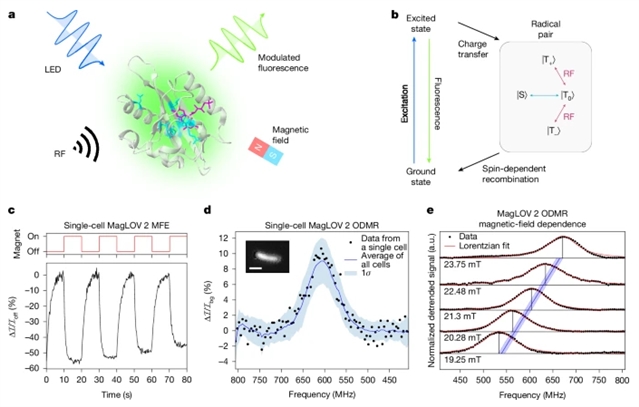
研究组最近开发了一类磁敏荧光蛋白,其中包括MagLOV,其克服了上述诸多挑战。研究表明,通过定向进化,可以改造这些蛋白质以改变其对磁场和射频的响应特性。研究组发现MagLOV在室温下的活细菌细胞内表现出光学探测磁共振信号,其信噪比足以实现单细胞检测。这些效应可通过自由基对机理加以解释,该机理涉及蛋白质骨架与结合的黄素辅因子。
利用光学探测磁共振和荧光磁场效应,研究组探索了一系列应用,包括使用梯度场实现荧光信号的空间定位(即利用基因编码探针进行磁共振成像)、分子微环境传感、生物成像多重化以及锁相检测,从而缓解了光散射和自发荧光等典型生物成像难题。总而言之,该研究成果代表了一套针对工程化生物系统的传感模式,其基于对磁敏荧光蛋白量子力学特性的理解并围绕此设计而成。
附:英文原文
Title: Quantum spin resonance in engineered proteins for multimodal sensing
Author: Abrahams, Gabriel, tuhec, Ana, Spreng, Vincent, Henry, Robin, Kempf, Idris, James, Jessica, Sechkar, Kirill, Stacey, Scott, Trelles-Fernandez, Vicente, Antill, Lewis M., Timmel, Christiane R., Miller, Jack J., Ingaramo, Maria, York, Andrew G., Tetienne, Jean-Philippe, Steel, Harrison
Issue&Volume: 2026-01-21
Abstract: Sensing technologies that exploit quantum phenomena for measurement are finding increasing applications across materials, physical and biological sciences1,2,3,4,5,6,7. Until recently, biological candidates for quantum sensors were limited to in vitro systems, had poor sensitivity and were prone to light-induced degradation. These limitations impeded practical biotechnological applications, and high-throughput study that would facilitate their engineering and optimization. We recently developed a class of magneto-sensitive fluorescent proteins including MagLOV, which overcomes many of these challenges8. Here we show that through directed evolution, it is possible to engineer these proteins to alter the properties of their response to magnetic fields and radio frequencies. We find that MagLOV exhibits optically detected magnetic resonance in living bacterial cells at room temperature, at sufficiently high signal-to-noise for single-cell detection. These effects are explained through the radical-pair mechanism, which involves the protein backbone and a bound flavin cofactor. Using optically detected magnetic resonance and fluorescence magnetic-field effects, we explore a range of applications, including spatial localization of fluorescence signals using gradient fields (that is, magnetic resonance imaging using a genetically encoded probe), sensing of the molecular microenvironment, multiplexing of bio-imaging and lock-in detection, mitigating typical biological imaging challenges such as light scattering and autofluorescence. Taken together, our results represent a suite of sensing modalities for engineered biological systems, based on and designed around understanding the quantum-mechanical properties of magneto-sensitive fluorescent proteins.
DOI: 10.1038/s41586-025-09971-3
Source: https://www.nature.com/articles/s41586-025-09971-3
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
