约翰霍普金斯大学医学院Erika L. Pearce团队的一项最新研究探明了从单核细胞到组织巨噬细胞的转变需要DHPS。2026年1月21日,国际知名学术期刊《自然》发表了这一成果。
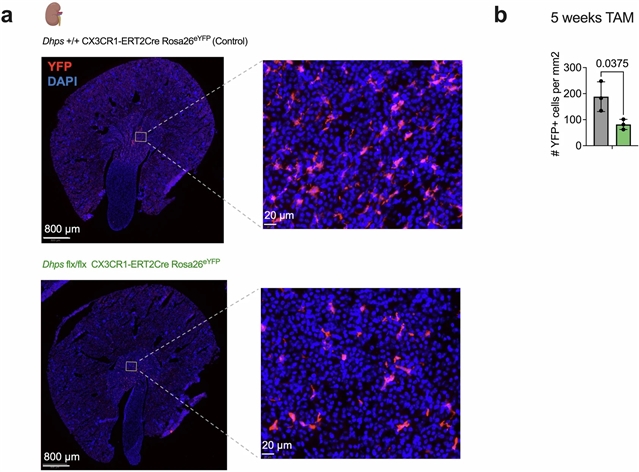
在这里,研究小组发现脱氧琥珀酰合酶(DHPS)是RTM分化和维持所必需的,DHPS是一种介导亚精胺依赖的翻译因子eif5a5,7的高琥珀酰修饰的酶。骨髓细胞缺乏DHPS的小鼠(DHPS -ΔM小鼠)在跨组织的RTMs中存在全局性缺陷,导致持续但最终无效的单核细胞内流。对DHPS缺陷巨噬细胞的转录分析表明,它们分化为成熟RTMs的能力受到阻碍,而蛋白质组学显示,细胞粘附和信号通路存在缺陷。核糖体参与转录物的测序鉴定了一组mRNAs,这些mRNAs参与细胞粘附和信号传导,依赖DHPS进行有效的翻译。组织中DHPS缺陷巨噬细胞的成像显示形态和组织相互作用的差异,这与它们的RTM分化失败有关。DHPS缺陷的巨噬细胞在关键的稳态RTM功能中也存在缺陷,包括传出细胞作用和组织维持。总之,他们的结果证明了一种细胞内在的、与组织无关的途径,可以驱动单核细胞来源的巨噬细胞分化为RTMs。
据了解,组织常驻巨噬细胞(RTMs)在胚胎发生过程中形成,局部自我更新,并通过清除死细胞和碎片来调节组织稳态。然而,在组织损伤期间,骨髓来源的单核细胞进入组织并分化为RTMs,修复组织并补充壁龛中的巨噬细胞。控制单核细胞向RTM转化和组织间成熟RTMs维持的普遍细胞内在机制仍不清楚。
附:英文原文
Title: The transition from monocyte to tissue-resident macrophage requires DHPS
Author: Carrizo, Gustavo E., Lin, Pianpian, Lee, Seung Hyun, Shenderov, Kevin, Blriot, Camille, Cha, Minsun, Schimmelpfennig, Lena, Shen, Zhen, van Teijlingen Bakker, Nikki, Grzes, Katarzyna M., Kelly, Beth, Safinia, Niloufar, Schole, Kate L., Musa, Yaarub, Mittler, Gerhard, Zen, Yoh, Pearce, Edward J., Ginhoux, Florent, Sanin, David E., Puleston, Daniel J., Pearce, Erika L., Carrizo, Gustavo E., Lin, Pianpian, Lee, Seung Hyun, Shenderov, Kevin, Blriot, Camille, Cha, Minsun, Schimmelpfennig, Lena, Shen, Zhen, van Teijlingen Bakker, Nikki, Grzes, Katarzyna M., Kelly, Beth, Safinia, Niloufar, Schole, Kate L., Musa, Yaarub, Mittler, Gerhard, Zen, Yoh, Pearce, Edward J., Ginhoux, Florent, Sanin, David E., Puleston, Daniel J., Pearce, Erika L.
Issue&Volume: 2026-01-21
Abstract: Tissue-resident macrophages (RTMs) form during embryogenesis, self-renew locally, and regulate tissue homeostasis by clearing dead cells and debris1,2,3,4,5,6. During tissue damage, however, bone-marrow-derived monocytes enter tissues and differentiate into RTMs, repairing the tissue and replenishing macrophages in the niche1. The universal cell-intrinsic mechanisms that control the monocyte-to-RTM transition and the maintenance of mature RTMs across tissues remain elusive3. Here we show that deoxyhypusine synthase (DHPS), an enzyme that mediates spermidine-dependent hypusine modification of translation factor eIF5A5,7, is required for RTM differentiation and maintenance. Mice with myeloid cell lack of DHPS (Dhps-ΔM mice) had a global defect in RTMs across tissues, resulting in persistent but ultimately futile monocyte influx. Transcriptional analyses of DHPS-deficient macrophages indicated a block in their ability to differentiate into mature RTMs, whereas proteomics revealed defects in cell adhesion and signalling pathways. Sequencing of ribosome-engaged transcripts identified a subset of mRNAs involved in cell adhesion and signalling that rely on DHPS for efficient translation. Imaging of DHPS-deficient macrophages in tissues showed differences in morphology and tissue interactions, which were correlated with their failed RTM differentiation. DHPS-deficient macrophages were also defective in critical homeostatic RTM functions including efferocytosis and tissue maintenance. Together, our results demonstrate a cell-intrinsic, tissue-agnostic pathway that drives differentiation of monocyte-derived macrophages into RTMs.
DOI: 10.1038/s41586-025-09972-2
Source: https://www.nature.com/articles/s41586-025-09972-2
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
