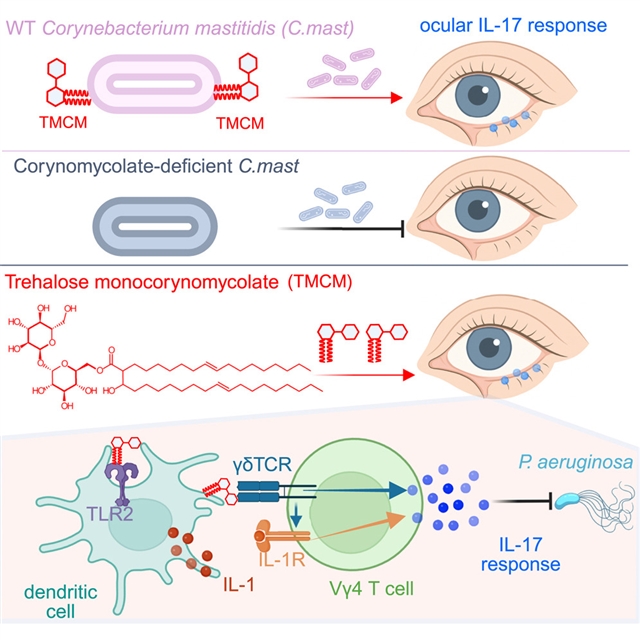
美国国立卫生研究院Rachel R. Caspi小组的一项最新研究发现了共生海藻糖引发γδ T细胞驱动的保护性眼屏障免疫。相关论文发表在2026年1月19日出版的《免疫学》杂志上。
该课题组人员从乳腺炎棒状杆菌中发现海藻糖单根藓酸盐(TMCM)是眼表Vγ4 γδ T细胞产生白细胞介素17 (IL-17)的有效刺激物。在机制上,TMCM诱导的IL-17反应依赖于IL-1R和γδ T细胞受体(TCR)信号传导,TCR参与进一步增强IL-1R1在γδ T细胞上的表达。单独合成的TMCM重现了乳腺炎棒状杆菌在眼表激发保护性γδ T细胞免疫以预防细菌感染的作用。
此外,TMCM还能促进下游排眼组织和皮肤的保护性免疫。这些发现确立了TMCM作为一种广泛适用的共体驱动免疫防御介质,并强调了其在加强IL-17介导的屏障部位保护方面的治疗潜力。
研究人员表示,共生体通过与宿主受体的分子串扰来塑造宿主生理。识别影响宿主免疫的特定微生物因子是理解宿主-微生物界面稳态和推进微生物治疗的关键。
附:英文原文
Title: Commensal-derived trehalose monocorynomycolate triggers γδ T cell-driven protective ocular barrier immunity
Author: Xiaoyan Xu, Yannis E. Rigas, Mary J. Mattapallil, Jing Guo, Keiko Sakamoto, Keisuke Nagao, Vijayaraj Nagarajan, Eric Bohrnsen, Crystal Richards, Akriti Gupta, Guillaume Gaud, Paul E. Love, Timothy Jiang, Amy Zhang, Biying Xu, Zixuan Peng, Yingyos Jittayasothorn, Mary A. Carr, M. Teresa Magone, Nathan T. Brandes, Jackie Shane, Benjamin Schwarz, Anthony J. St. Leger, Rachel R. Caspi
Issue&Volume: 2026-01-19
Abstract: Commensals shape host physiology through molecular crosstalk with host receptors. Identifying specific microbial factors that causally influence host immunity is key to understanding homeostasis at the host-microbe interface and advancing microbial-based therapeutics. Here, we identified trehalose monocorynomycolate (TMCM) from Corynebacterium mastitidis as a potent stimulator of interleukin 17 (IL-17) production by Vγ4 γδ T cells at the ocular surface. Mechanistically, TMCM-induced IL-17 responses depended on IL-1R and γδ T cell receptor (TCR) signaling, with TCR engagement further enhancing IL-1R1 expression on γδ T cells. Synthetic TMCM alone recapitulated the effect of Corynebacterium mastitidis in eliciting protective γδ T cell immunity at the ocular surface to prevent bacterial infection. Moreover, TMCM also promoted protective immunity in downstream eye-draining tissues and skin. These findings establish TMCM as a broadly applicable mediator of commensal-driven immune defense and highlight its therapeutic potential to strengthen IL-17-mediated protection at barrier sites.
DOI: 10.1016/j.immuni.2025.12.007
Source: https://www.cell.com/immunity/abstract/S1074-7613(25)00563-1
Immunity:《免疫》,创刊于1994年。隶属于细胞出版社,最新IF:43.474
官方网址:https://www.cell.com/immunity/home
投稿链接:https://www.editorialmanager.com/immunity/default.aspx
