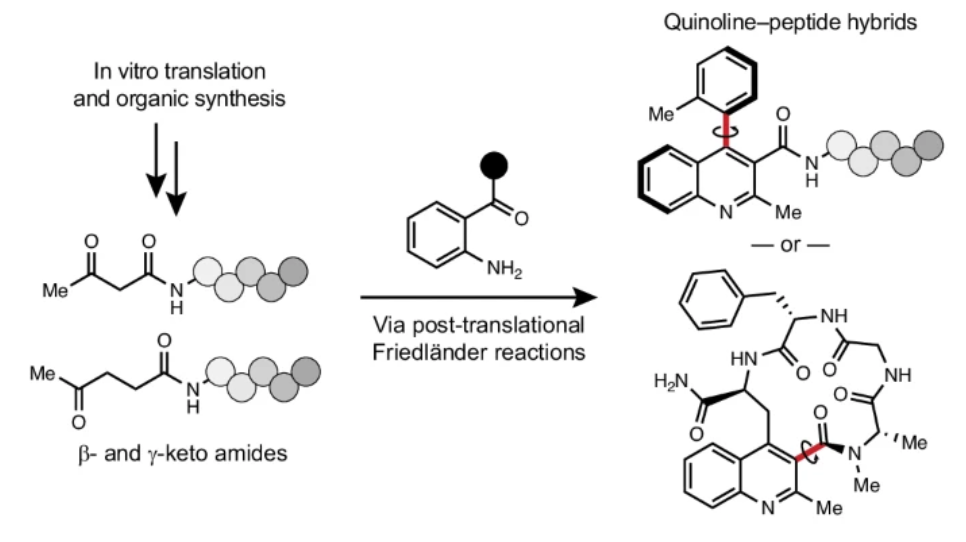
近日,美国耶鲁大学Miller, Scott J.团队研究了具有嵌入式喹啉的阻转异构和大环肽的化学合成与核糖体合成。2025年9月17日,《自然-化学》杂志发表了这一成果。
筛选含有非规范氨基酸和大环结构的核糖体合成肽的数万亿成员文库,定期返回治疗相关靶标的有效肽配体。然而,这些肽所探索的化学空间只是天然产物和药物所体现的一小部分,大多数肽先导物需要彻底的药物化学优化来提高效力和物理化学。
为了解决在肽大环中引入化学复杂性和构象控制策略的需要,研究组报道了具有反应性N端β-酮或γ-酮酰胺的线性肽可以通过核糖体合成。后续的弗里德兰德反应生成了喹啉-肽杂合分子,其中部分分子含有稳定的联芳基阻旋异构轴。研究组还展示了分子内弗里德兰德大环化反应——该反应条件足够温和,可直接应用于未受保护且经体外翻译的肽链——将喹啉药效团直接嵌入肽骨架中。通过将N末端酮基 motif 引入基因编码材料并进行翻译后衍生化,为程序化合成更近似复杂天然产物的肽衍生材料提供了新范式。
附:英文原文
Title: Chemical and ribosomal synthesis of atropisomeric and macrocyclic peptides with embedded quinolines
Author: Knudson, Isaac J., Dover, Taylor L., Dilworth, Diondra A., Paloutzian, Cameron, Paz, Orel, Lin, Jieye, Cho, Hannah, Gonen, Tamir, Schepartz, Alanna, Miller, Scott J.
Issue&Volume: 2025-09-17
Abstract: Potent peptide ligands for therapeutically relevant targets are regularly returned from screening trillion-member libraries of ribosomally synthesized peptides containing non-canonical amino acids and macrocyclic architectures. Yet the chemical space explored by these peptides is a fraction of that embodied by natural products and pharmaceuticals, and most peptide leads require exhaustive medicinal chemistry optimization to improve potency and physicochemistry. To address the need for strategies to introduce chemical complexity and conformational control into peptide macrocycles, we report here that linear peptides with a reactive N-terminal β-keto or γ-keto amide can be synthesized ribosomally. Subsequent Friedlnder reactions generate quinoline–peptide hybrids, some of which contain stable biaryl atropisomeric axes. We also demonstrate intramolecular Friedlnder macrocyclization reactions—sufficiently mild to be employed on unprotected and in vitro-translated peptides—that embed a quinoline pharmacophore directly within the peptide backbone. The introduction of N-terminal ketone motifs into genetically encoded materials and their post-translational derivatization provides a paradigm for the programmed synthesis of peptide-derived materials that more closely resemble complex natural products.
DOI: 10.1038/s41557-025-01935-4
Source: https://www.nature.com/articles/s41557-025-01935-4
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
