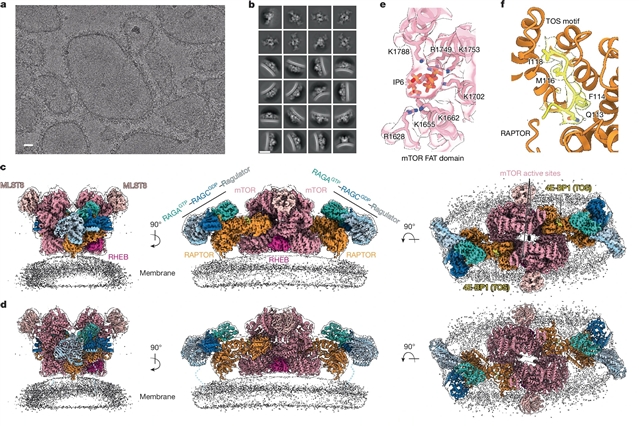
美国加州大学James H. Hurley团队的研究开发出了mTORC1在溶酶体膜上活化的结构基础。相关论文于2025年9月17日发表在《自然》杂志上。
该研究团队用RHEB、RAGs和Ragulator重组了mTORC1在细胞膜上的活化。低温电子显微镜显示RAPTOR和mTOR直接与膜相互作用。膜锚的充分参与是mTOR激酶活性位点催化残基的最佳定位所必需的。来自GFs和营养物质的信号聚集驱动mTORC1在溶酶体膜上募集并激活,这是一个四步过程,包括(1)RAG–Ragulator驱动的募集到~100溶酶体膜的Å;(2) RHEB-驱动的招聘在~40Å;(3) RAPTOR-膜结合和中间酶活化;(4) mTOR-膜结合和全酶激活。RHEB和膜结合导致充分的催化激活,并在结构上解释了GF和营养信号在溶酶体中的整合。
据了解,雷帕霉素复合物1 (mTORC1)的机制靶点整合生长因子(GF)和营养信号,刺激与细胞生长相关的合成代谢过程,抑制自噬等分解代谢过程。GF信号通过结节硬化复合体调节溶酶体定位的小GTPase RAS同源物富集于大脑(RHEB)。RHEB-GTP直接与mTORC1的mTOR激酶亚基结合,通过诱导大规模构象变化而变构激活该激酶。
附:英文原文
Title: Structural basis for mTORC1 activation on the lysosomal membrane
Author: Cui, Zhicheng, Esposito, Alessandra, Napolitano, Gennaro, Ballabio, Andrea, Hurley, James H.
Issue&Volume: 2025-09-17
Abstract: The mechanistic target of rapamycin complex 1 (mTORC1) integrates growth factor (GF) and nutrient signals to stimulate anabolic processes connected to cell growth and inhibit catabolic processes such as autophagy1,2. GF signalling through the tuberous sclerosis complex regulates the lysosomally localized small GTPase RAS homologue enriched in brain (RHEB)3. Direct binding of RHEB–GTP to the mTOR kinase subunit of mTORC1 allosterically activates the kinase by inducing a large-scale conformational change4. Here we reconstituted mTORC1 activation on membranes by RHEB, RAGs and Ragulator. Cryo-electron microscopy showed that RAPTOR and mTOR interact directly with the membrane. Full engagement of the membrane anchors is required for optimal alignment of the catalytic residues in the mTOR kinase active site. Converging signals from GFs and nutrients drive mTORC1 recruitment to and activation on lysosomal membrane in a four-step process, consisting of (1) RAG–Ragulator-driven recruitment to within ~100 Å of the lysosomal membrane; (2) RHEB-driven recruitment to within ~40 Å ; (3) RAPTOR–membrane engagement and intermediate enzyme activation; and (4) mTOR–membrane engagement and full enzyme activation. RHEB and membrane engagement combined leads to full catalytic activation and structurally explains GF and nutrient signal integration at the lysosome.
DOI: 10.1038/s41586-025-09545-3
Source: https://www.nature.com/articles/s41586-025-09545-3
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
