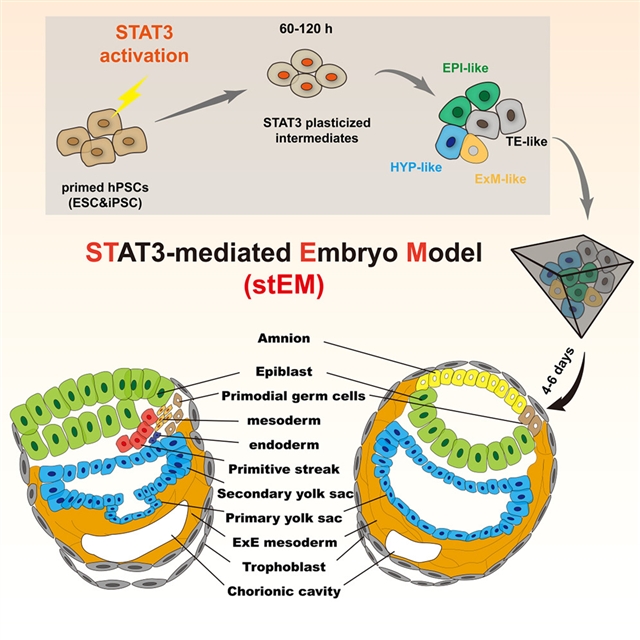
通过Stat3激活的信号重编程揭示了高保真的人类植入后胚胎建模,这一成果由广州国家实验室José C.R. Silva团队经过不懈努力而取得。相关论文于2025年9月16日发表于国际顶尖学术期刊《细胞—干细胞》杂志上。
课题组研究STAT3激活是否可以重编程多能干细胞(PSCs)进入自组织成胚胎模型的早期命运。使用增强STAT3活性(SAM)的培养基,PSCs在60小时内重编程为下胚层、滋养外胚层、原始外胚层和胚外中胚层。在60-120 h解离经过SAM处理的PSCs,然后进行3D培养,可获得植入后胚胎样结构的动态发育,效率高达52.41%±8.92%。第6天的样品类似于卡内基5期(CS5)至7期(CS7)胚胎,表现出双层盘状结构,包括外胚层和卵黄囊、羊膜腔、间充质、绒毛膜腔和滋养细胞。值得注意的是,CS6/7样样本显示原肠胚形成,包括原始条纹的形成和正确定位、上皮向间质转变、中胚层和终胚层。STAT3介导的胚胎模型在分子上也与CS6/7胚胎文献密切一致,代表了推进人类胚胎发生研究的最先进平台。
据悉,人类胚胎模型在推进医学方面有着巨大的希望,但目前的系统在复制植入后阶段缺乏效率和保真度。
附:英文原文
Title: Signaling reprogramming via Stat3 activation unravels high-fidelity human post-implantation embryo modeling
Author: Chuanxin Chen, Jinyi Wu, Xinggu Wang, Litao Chang, Kexin Wang, Kaiyi Wu, Mingyue Guo, Huanhuan Li, Fei Sun, Xinxing Jiang, Yanlin Ma, Guangjin Pan, Zhenyu Xiao, José C.R. Silva
Issue&Volume: 2025-09-16
Abstract: Human embryo models hold great promise for advancing medicine, but current systems lack efficiency and fidelity in replicating post-implantation stages. Here, we investigate whether STAT3 activation can reprogram pluripotent stem cells (PSCs) into early fates that self-organize into embryo models. Using a medium enhancing STAT3 activity (SAM), PSCs reprogram within 60 h into hypoblast, trophectoderm, naive epiblast, and extraembryonic mesoderm. Dissociating SAM-treated PSCs at 60–120 h, followed by 3D culture, results in dynamic development of post-implantation embryo-like structures with up to 52.41% ± 8.92% efficiency. Resulting day 6 examples resemble Carnegie stages 5 (CS5) to 7 (CS7) embryos, exhibiting bilaminar disc structure with epiblast and yolk sac, amniotic cavity, mesenchyme, chorionic cavity, and trophoblast. Notably, CS6/7-like examples exhibit gastrulation, including the formation and correct positioning of primitive streak, epithelial-to-mesenchymal transition, mesoderm, and definitive endoderm. The STAT3-mediated embryo model also closely aligns molecularly with CS6/7 embryo references and represents a state-of-the-art platform for advancing human embryogenesis research.
DOI: 10.1016/j.stem.2025.08.011
Source: https://www.cell.com/cell-stem-cell/abstract/S1934-5909(25)00303-0
Cell Stem Cell:《细胞—干细胞》,创刊于2007年。隶属于细胞出版社,最新IF:25.269
官方网址:https://www.cell.com/cell-stem-cell/home
投稿链接:https://www.editorialmanager.com/cell-stem-cell/default.aspx
