近日,重庆师范大学李泓霖团队揭示了Fe3C纳米簇调控电荷不对称铁双原子位增强氧还原性能。2025年8月19日,《中国化学》杂志发表了这一成果。
双原子催化剂(DAC)在氧还原反应(ORR)中具有良好的应用前景,但在平衡活性和耐久性的精确电荷调制方面仍面临挑战。
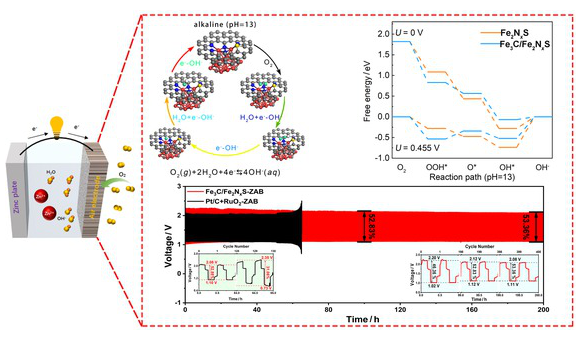
研究组通过热解ZIF-8包封铁二聚体和掺硫C3N4的混合物,制备了一种由Fe3C纳米微米修饰的N、S配位铁双原子催化剂(Fe3C/Fe2NxS)。经像差校正的STEM和同步X射线吸收光谱(XAS)验证,催化剂由Fe双原子位和Fe3C纳米粒子组成,其中Fe双原子由5个N原子和1个S原子配位。Fe3C/Fe2NxS在碱性介质中表现出优异的ORR活性,表现出高半波电位(E1/2 = - 0.894 V vs. RHE)和近4e-选择性(n = 3.92),在20000 s后保持86.8%的保留率,优于商用Pt/C。
令人印象深刻的是,组装的锌空气电池提供了卓越的峰值功率密度(163 mW·cm-2)和200小时的电池稳定性。密度泛函理论(DFT)计算表明,电子从Fe2NxS的Fe转移到邻近的Fe3C引起了局部电荷不对称,使d带中心更靠近费米能级,从而增强了O2的活化。此外,Fe3C/Fe2NxS中的OOH*形成能垒降低到0.52 eV,加速了ORR反应动力学。这项工作建立了纳米热介导的电子再分配,以适应高性能电催化剂的电荷不对称。
附:英文原文
Title: Fe3C Nanoclusters Regulated Charge Asymmetric Fe Dual Atomic Sites for Enhanced Oxygen Reduction Performance
Author: Ying Lei, Jiaxin Li, Yi Cheng, Chuanlan Xu, Honglin Li
Issue&Volume: 2025-08-19
Abstract: Dual-atom catalysts (DACs) show attractive prospects for the oxygen reduction reaction (ORR), yet face challenges in precise charge modulation that balances the activity and durability. Herein, we present a N,S-coordinated Fe dual atomic catalyst modified by Fe3C nanoclusters (Fe3C/Fe2NxS) through pyrolyzing the mixtures of ZIF-8-encapsulated iron dimers and sulfur-doped C3N4. Aberration-corrected STEM and synchrotron X-ray absorption spectroscopy (XAS) validated that the catalyst was composed of Fe dual atomic sites and Fe3C nanoclusters, in which Fe dual atoms were coordinated by five N atoms and one S atom. Fe3C/Fe2NxS exhibited excellent ORR activity in alkaline media, displaying a high half-wave potential (E1/2 =0.894 V vs. RHE) with near 4e– selectivity (n =3.92) and maintaining 86.8% retention after 20000 s, superior to commercial Pt/C. Impressively, the assembled zinc-air battery delivered exceptional peak power density (163 mW·cm–2) and 200-hour robust stability. Density functional theory (DFT) calculations revealed that electron transfer from Fe of Fe2NxS to neighboring Fe3C induced local charge asymmetry, shifting the d-band center closer to Fermi level, thereby enhancing O2 activation. Moreover, the OOH* formation energy barrier was reduced to 0.52 eV in Fe3C/Fe2NxS, accelerating ORR reaction kinetics. This work establishes nanocluster-mediated electronic redistribution to tailor charge asymmetry for high-performance electrocatalysts.
DOI: 10.1002/cjoc.70221
Source: https://onlinelibrary.wiley.com/doi/10.1002/cjoc.70221
Chinese Journal of Chemistry:《中国化学》,创刊于1983年。隶属于Wiley,最新IF:5.4
官方网址:https://onlinelibrary.wiley.com/journal/16147065
投稿链接:https://mc.manuscriptcentral.com/cjoc
