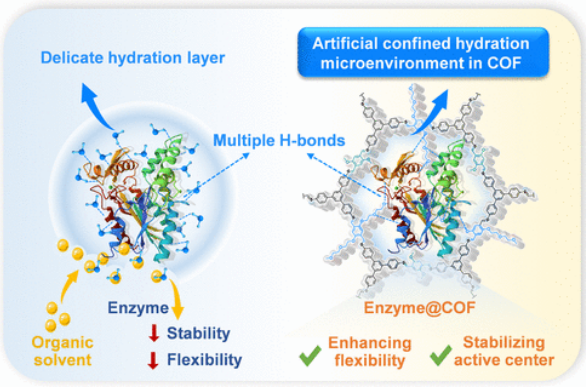
近日,北京理工大学王博团队实现了在共价有机框架中定制人工水化微环境以增强有机介质中的酶催化作用
生物界面的水合作用通过稳定结构和驱动催化所需的构象动力学在酶的功能中起着至关重要的作用。然而,在有机溶剂中保存这些微妙的水合微环境仍然具有挑战性,这破坏了非水酶催化并阻碍了体内生物催化的应用。
研究组通过将富含氢键受体的柔性低聚(环氧乙烷)链整合到共价有机框架(COFs)的受限通道中,为酶量身定制人工水合微环境。这些工程微环境促进了被封装酶与孔壁之间的多重氢键相互作用,显著提高了酶在有机介质中的活性和稳定性。值得注意的是,在0%相对湿度(RH)条件下,包封酶的转化率比游离酶高约13倍,同时在较宽的湿度范围内保持较高的性能。
与脆弱的天然水合层不同,这种在受限纳米通道内设计的水合环境是稳定的,在极性溶剂中有效地保持酶的活性,同时也能抵抗高温。分子动力学(MD)模拟表明,多重氢键介导的微环境增强了酶的局部构象灵活性,稳定了酶的催化活性中心。此外,这种方法的多功能性在脂肪酶催化的活性药物中间体的区域选择性合成中得到了证明。该研究为构建机器人水合微环境建立了有效的策略,促进了非水系统中高效生物催化剂的设计。
附:英文原文
Title: Tailoring Artificial Hydration Microenvironments in Covalent Organic Frameworks for Enhanced Enzymatic Catalysis in Organic Media
Author: Chunyan Xing, Zhenjie Mu, Bixiao Li, Jianwei Yang, Xiao Feng, Yuanyuan Zhang, Bo Wang
Issue&Volume: August 11, 2025
Abstract: Hydration at biological interfaces plays a crucial role in enzyme function by stabilizing structures and driving conformational dynamics essential for catalysis. However, preserving these delicate hydration microenvironments in organic solvents remains challenging, undermining nonaqueous enzymatic catalysis and impeding industrial biocatalytic applications. Here, we tailored artificial hydration-like microenvironments for enzymes by integrating flexible oligo(ethylene oxide) chains, rich in hydrogen-bonding acceptors, into the confined channels of covalent organic frameworks (COFs). These engineered microenvironments promote multiple hydrogen-bond interactions between the encapsulated enzyme and the pore walls, significantly enhancing enzyme activity and stability in organic media. Notably, the encapsulated enzyme exhibited approximately 13-fold higher conversion than the free enzyme under 0% relative humidity (RH), while maintaining high performance across a wide humidity range. Unlike the fragile natural hydration layer, this hydration-like environment engineered within the confined nanochannels is stable, effectively preserving enzyme activity in polar solvents, while also resisting elevated temperature. Molecular dynamics (MD) simulations reveal that the multiple hydrogen bond-mediated microenvironment enhances the local conformational flexibility of enzyme and stabilizes its catalytic active center. Furthermore, the versatility of this approach is demonstrated in the lipase-catalyzed regioselective synthesis of active pharmaceutical intermediates. This work establishes an effective strategy for constructing robust hydration-like microenvironments, advancing the design of efficient biocatalysts in nonaqueous systems.
DOI: 10.1021/jacs.5c07600
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c07600
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
