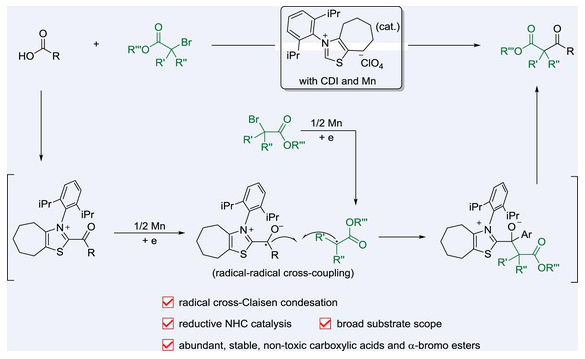
近日,中国海洋大学王洪玉团队实现了NHC催化自由基交叉缩合合成多种β-酮酯。2025年8月7日出版的《中国化学》杂志发表了这项成果。
交叉克莱森缩合是合成β-酮酯的一种有价值的方法,但传统上受限于需要强碱或预成型的硅基酮缩醛(SKAs)或底物范围狭窄。
为了解决这些问题,研究组在酰基咪唑和α-溴酯之间建立了一种温和的N-杂环碳(NHC)催化的自由基交叉克莱森缩合反应。该方案采用Mn介导的单电子还原生成持久的酮基和α-羰基自由基,使两种亲电试剂在氧化还原-中性条件下有效交叉偶联。该反应具有广泛的官能团耐受性,可以直接从羧酸中进行一锅反应,为β-酮酯的合成提供了一个实用的平台。
附:英文原文
Title: NHC-Catalyzed Radical Cross-Claisen Condensation for Diverse β-Keto Ester Synthesis
Author: Xiangyu Zhuang, Hao Li, Hongyu Wang
Issue&Volume: 2025-08-07
Abstract: The cross-Claisen condensation is a valuable method for synthesizing β-keto esters but is traditionally limited by the need for strong bases or preformed silyl ketene acetals (SKAs) or a narrow substrate scope. To address these challenges, we developed a mild, N-heterocyclic carbene (NHC)-catalyzed radical cross-Claisen condensation between acyl imidazoles and α-bromo esters. This protocol employs Mn-mediated single-electron reduction to generate persistent ketyl and α-carbonyl radicals, enabling efficient cross-coupling of two electrophiles under redox-neutral conditions. The reaction proceeds with broad functional-group tolerance and can be conducted in a one-pot manner directly from carboxylic acids, offering a practical platform for β-keto ester synthesis.
DOI: 10.1002/cjoc.70214
Source: https://onlinelibrary.wiley.com/doi/10.1002/cjoc.70214
Chinese Journal of Chemistry:《中国化学》,创刊于1983年。隶属于Wiley,最新IF:5.4
官方网址:https://onlinelibrary.wiley.com/journal/16147065
投稿链接:https://mc.manuscriptcentral.com/cjoc
