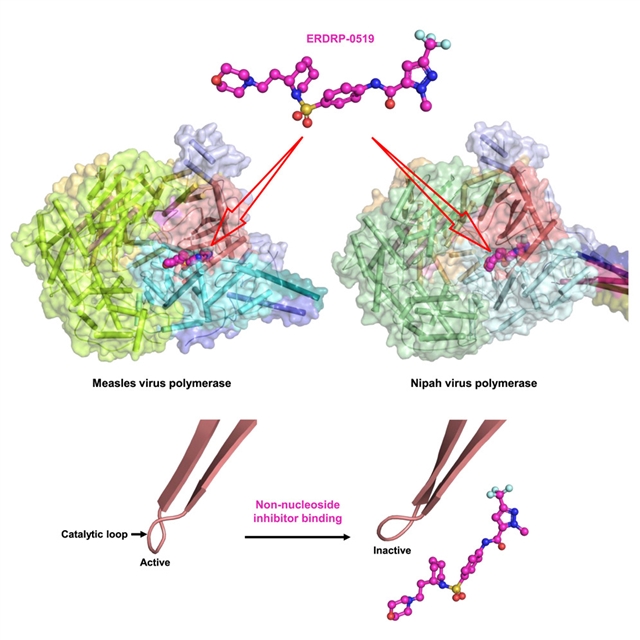
上海科技大学张贺桥小组取得一项新突破。他们提出了麻疹病毒非核苷抑制剂聚合酶复合物的结构及其抑制机制。相关论文发表在2025年7月7日出版的《细胞》杂志上。
研究小组测定了MeV聚合酶复合物单独和结合两种非核苷抑制剂ERDRP-0519和AS-136A的低温电镜结构。抑制剂结合诱导催化环的构象变化,变构锁定聚合酶处于非活性的“GDN-out”状态。这些发现促使人们提出,ERDRP-0519也可能对尼帕病毒(Nipah virthem, NiV)有效,尼帕病毒是一种没有可用抗病毒药物的高致病性病毒。这一建议被NiV聚合酶复合物的结构测定和转录抑制所证实。
研究人员表示,麻疹病毒(MeV)是副粘病毒科的一种高度传染性的非节段负义RNA病毒,每年导致数百万人感染,目前尚无批准的抗病毒药物可用。由大(L)蛋白和四聚体磷蛋白(P)组成的病毒聚合酶复合物是一个关键的抗病毒靶点。
附:英文原文
Title: Structures of the measles virus polymerase complex with non-nucleoside inhibitors and mechanism of inhibition
Author: Yiru Wang, Lixia Zhao, Yi Zhang, Xiuxia Gao, Yannan Wang, Wenping Shi, Roger D. Kornberg, Heqiao Zhang
Issue&Volume: 2025-07-07
Abstract: The measles virus (MeV), a highly contagious non-segmented negative-sense RNA virus in the Paramyxoviridae family, causes millions of infections annually, with no approved antivirals available. The viral polymerase complex, comprising the large (L) protein and the tetrameric phosphoprotein (P), is a key antiviral target. We determined the cryo-electron microscopy structures of the MeV polymerase complex alone and bound to two non-nucleoside inhibitors, ERDRP-0519 and AS-136A. Inhibitor binding induces a conformational change in the catalytic loop, allosterically locking the polymerase in an inactive “GDN-out” state. These findings led to the proposal that ERDRP-0519 would also be effective against Nipah virus (NiV), a highly pathogenic virus with no available antivirals. This proposal was confirmed by structure determination of the NiV polymerase complex and by inhibition of transcription.
DOI: 10.1016/j.cell.2025.06.017
Source: https://www.cell.com/cell/abstract/S0092-8674(25)00683-X
