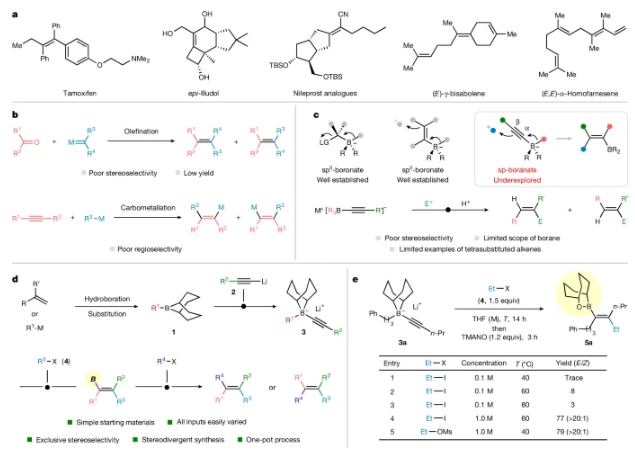
近日,英国布里斯托大学Aggarwal, Varinder K.团队实现了硼介导的四取代烯烃模块化组装。该研究于2025年7月2日发表在《自然》杂志上。
烯烃是有机化学的核心部分。然而,尽管大多数烯烃易于制备,但四取代烯烃的受控合成仍然具有挑战性,这些烯烃在中心C=C键周围有四个基团。
研究组报告了硼介导的四取代烯烃的组装,完全控制了双键的几何形状。迁移基团和亲电试剂在炔烃上添加顺式。温和的氧化会产生中间体硼酸酯,可以分离和纯化,也可以直接在一系列转化中反应,包括交叉偶联和同系反应。特别是,使中间体硼酸酯经受Zweifel烯化条件可以保留或反转双键几何形状,这取决于是否存在碱。
较易获得四取代烯烃的不同位置和立体异构体,突出了该方法的广度和通用性。这通过其成功应用于高产率和立体控制的药物分子和天然产物的快速合成中得到了证明。这种方法不仅有效地解决了四取代烯烃立体控制合成的长期挑战,还引入了与非经典硼离子在Zweifel烯化反应中的干预相关的新概念。
附:英文原文
Title: Boron-mediated modular assembly of tetrasubstituted alkenes
Author: Wei, Liang, Popescu, Mihai V., Noble, Adam, Paton, Robert S., Aggarwal, Varinder K.
Issue&Volume: 2025-07-02
Abstract: Alkenes are a central part of organic chemistry1,2,3. However, although most alkenes are easy to prepare, the controlled synthesis of tetrasubstituted alkenes, those with four groups around the central C=C bond, remains challenging1,2,3,4,5. Here we report the boron-mediated assembly of tetrasubstituted alkenes with complete control of the double-bond geometry. The migrating group and electrophile add syn across the alkyne. Mild oxidation leads to intermediate borinic esters6, which can be isolated and purified or reacted directly in a range of transformations, including cross-couplings and homologation reactions. In particular, subjecting the intermediate borinic esters to Zweifel7,8 olefination conditions can give either retention or inversion of the double-bond geometry, depending on whether base is present or not. Different positional and stereoisomers of the tetrasubstituted alkenes can be easily accessed, highlighting the breadth and versatility of the method. This was showcased through its successful application to the rapid synthesis of drug molecules and natural products with high yield and stereocontrol. Not only does this method provide efficient access to the long-standing challenge of the stereocontrolled synthesis of tetrasubstituted alkenes but it also introduces new concepts related to the intervention of non-classical borenium ions in the Zweifel olefination.
DOI: 10.1038/s41586-025-09209-2
Source: https://www.nature.com/articles/s41586-025-09209-2
官方网址:http://www.nature.com/
