麦克马斯特大学Gregory R. Steinberg小组的最新研究提出了抑制ACLY可促进肿瘤免疫,抑制肝癌。这一研究成果于2025年7月30日发表在国际顶尖学术期刊《自然》上。
免疫抑制肿瘤微环境在代谢功能障碍相关脂肪性肝炎(MASH)驱动的肝细胞癌(HCC)(MASH-HCC)等癌症中很常见。虽然免疫细胞代谢影响效应体功能,但肿瘤代谢对免疫原性的影响尚不清楚。
ATP柠檬酸裂解酶(ACLY)将底物利用率和线粒体代谢与脂质生物合成和基因调控联系起来。尽管ACLY抑制在多种肿瘤中显示出抗增殖作用,但由于抑制剂发展和代偿代谢途径的挑战,临床转化受到限制。这是一个反映人类疾病的MASH-HCC的单主题模型,肝细胞和肿瘤中ACLY的遗传抑制使肿瘤病变减少了70%以上。
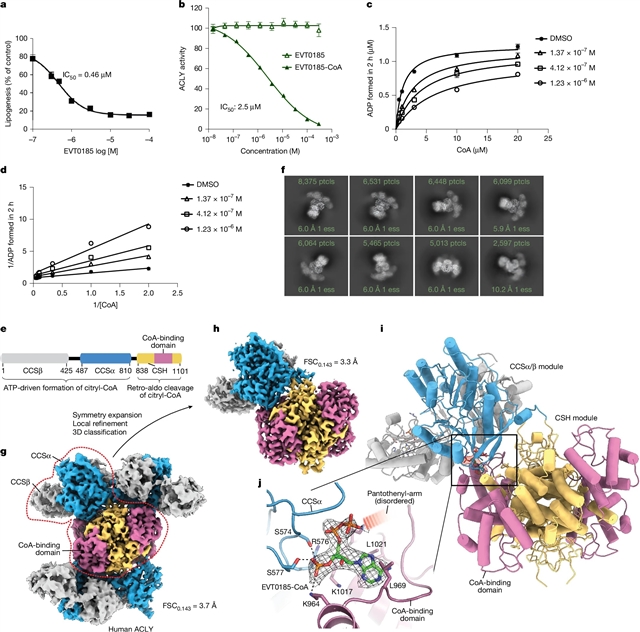
为了评估这一途径的治疗潜力,通过表型筛选鉴定了一种新型小分子ACLY抑制剂EVT0185(6-[4-(5-羧基-5-甲基己基)-苯基]2,2-二甲基己酸)。EVT0185在肝脏中被SLC27A2转化为CoA硫酯,低温电镜结构分析显示EVT0185-CoA直接与ACLY的CoA结合位点相互作用。口服EVT0185治疗三种MASH-HCC单药治疗可显著减轻肿瘤负担,并提高包括酪氨酸激酶抑制剂和免疫疗法在内的当前标准治疗的疗效。小鼠和人类的转录组学和空间分析表明,肿瘤ACLY的降低与趋化因子CXCL13、肿瘤浸润B细胞和三级淋巴样结构的增加有关。B细胞的消耗阻断了ACLY抑制的抗肿瘤作用。
总之,这些发现阐明了靶向肿瘤代谢如何在MASH-HCC中重塑免疫功能并抑制癌症进展。
附:英文原文
Title: ACLY inhibition promotes tumour immunity and suppresses liver cancer
Author: Gautam, Jaya, Wu, Jianhan, Lally, James S. V., McNicol, Jamie D., Fayyazi, Russta, Ahmadi, Elham, Oniciu, Daniela Carmen, Heaton, Spencer, Newton, Roger S., Rehal, Sonia, Bhattacharya, Dipankar, Di Pastena, Fiorella, Nguyen, Binh, Valvano, Celina M., Townsend, Logan K., Banskota, Suhrid, Batchuluun, Battsetseg, Jabile, Maria Joy Therese, Payne, Alice, Lu, Junfeng, Desjardins, Eric M., Kubota, Naoto, Tsakiridis, Evangelia E., Mistry, Bejal, Aganostopoulos, Alex, Houde, Vanessa, Dansercoer, Ann, Verschueren, Koen H. G., Savvides, Savvas N., Hammill, Joanne A., Bezverbnaya, Ksenia, Muti, Paola, Tsakiridis, Theodoros, Dai, Wenting, Jiang, Lei, Hoshida, Yujin, Larch, Mark, Bramson, Jonathan L., Friedman, Scott L., Verstraete, Kenneth, Wang, Dongdong, Steinberg, Gregory R.
Issue&Volume: 2025-07-30
Abstract: Immunosuppressive tumour microenvironments are common in cancers such as metabolic dysfunction-associated steatohepatitis (MASH)-driven hepatocellular carcinoma (HCC) (MASH-HCC)1,2,3. Although immune cell metabolism influences effector function, the effect of tumour metabolism on immunogenicity is less understood4. ATP citrate lyase (ACLY) links substrate availability and mitochondrial metabolism with lipid biosynthesis and gene regulation5,6,7. Although ACLY inhibition shows antiproliferative effects in various tumours, clinical translation has been limited by challenges in inhibitor development and compensatory metabolic pathways8,9,10,11,12. Here, using a mouse model of MASH-HCC that mirrors human disease, genetic inhibition of ACLY in hepatocytes and tumours reduced neoplastic lesions by over 70%. To evaluate the therapeutic potential of this pathway, a novel small-molecule ACLY inhibitor, EVT0185 (6-[4-(5-carboxy-5-methyl-hexyl)-phenyl]2,2-dimethylhexanoic acid), was identified via phenotypic screening. EVT0185 is converted to a CoA thioester in the liver by SLC27A2 and structural analysis by cryo-electron microscopy reveals that EVT0185-CoA directly interacts with the CoA-binding site of ACLY. Oral delivery of EVT0185 in three mouse models of MASH-HCC dramatically reduces tumour burden as monotherapy and enhances efficacy of current standards of care including tyrosine kinase inhibitors and immunotherapies. Transcriptomic and spatial profiling in mice and humans linked reduced tumour ACLY with increases in the chemokine CXCL13, tumour-infiltrating B cells and tertiary lymphoid structures. The depletion of B cells blocked the antitumour effects of ACLY inhibition. Together, these findings illustrate how targeting tumour metabolism can rewire immune function and suppress cancer progression in MASH-HCC.
DOI: 10.1038/s41586-025-09297-0
Source: https://www.nature.com/articles/s41586-025-09297-0
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
