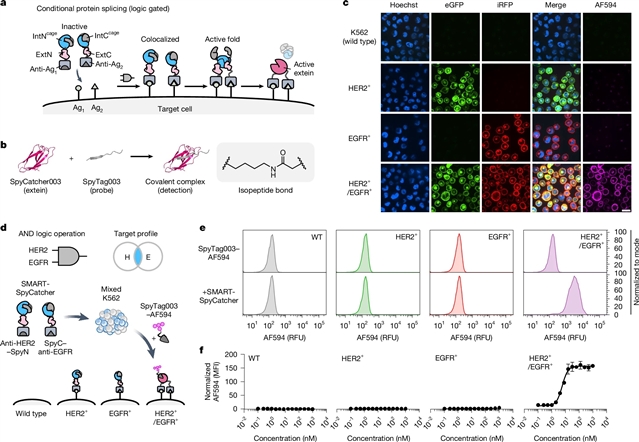
普林斯顿大学Tom W. Muir团队宣布他们开发出细胞表面的可编程蛋白连接。相关论文发表在2025年7月30日出版的《自然》杂志上。
在这里,该研究组描述了一种由邻近门控蛋白反式剪接驱动的自主决策装置,该装置允许从两个非活性多肽片段中局部生成活性蛋白。该课题组展示了这种蛋白质致动器平台可以在指定的细胞表面上执行会聚蛋白连接,允许高度选择性地生成活性蛋白,这些活性蛋白可以与制造它们的细胞表面保持物理关联,也可以释放到周围环境中。由于其固有的模块化和可调性,课题组研究人员证明该技术与不同类型的输入、目标模式和功能输出兼容,允许对细胞系统进行局部查询或操作。
据了解,细胞的表面景观因细胞类型的不同而不同,并且在疾病环境中经常发生改变。利用这些差异是许多治疗策略的关键,也是开发诊断和基础科学工具的基础。最先进的策略通常针对单个表面抗原,但每个受体很少定义特定的细胞类型。因此,开发集成多种细胞表面特征的可编程分子系统以将靶输入转换为热定义输出是非常可取的。
附:英文原文
Title: Programmable protein ligation on cell surfaces
Author: Kofoed, Christian, Erkalo, Girum, Tay, Nicholas E. S., Ye, Xuanjia, Lin, Yutong, Muir, Tom W.
Issue&Volume: 2025-07-30
Abstract: The surface landscapes of cells differ as a function of cell type and are frequently altered in disease contexts1,2,3. Exploiting such differences is key to many therapeutic strategies and is the basis for developing diagnostic and basic-science tools. State-of-the-art strategies typically target single surface antigens, but each individual receptor rarely defines the specific cell type4,5. The development of programmable molecular systems that integrate multiple cell-surface features to convert on-target inputs to user-defined outputs is therefore highly desirable. Here we describe an autonomous decision-making device driven by proximity-gated protein trans-splicing that allows local generation of an active protein from two otherwise inactive polypeptide fragments. We show that this protein-actuator platform can perform convergent protein ligation on designated cell surfaces, allowing highly selective generation of active proteins, which can either remain physically associated with the cell surface on which they were manufactured or be released into the surrounding milieu. Because of its intrinsic modularity and tunability, we demonstrate that the technology is compatible with different types of input, targeting modality and functional output, allowing for the localized interrogation or manipulation of cellular systems.
DOI: 10.1038/s41586-025-09287-2
Source: https://www.nature.com/articles/s41586-025-09287-2
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
