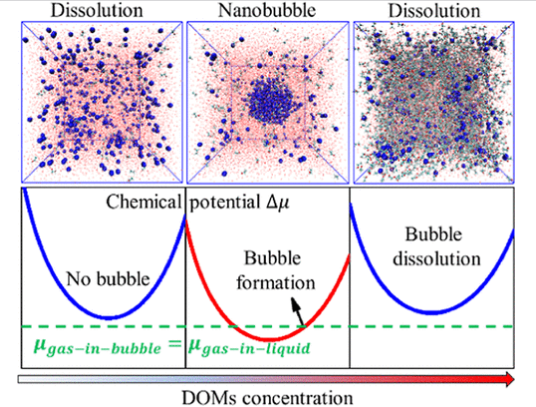
先前的实验表明,当水与可溶解的有机分子(DOMs)混合时,会产生不溶性的含气体的纳米材料。然而,对含气体纳米计的分子认识仍然缺乏。
研究组首次利用电中性界面模型,通过大规模模拟展示了水中不溶性气体与溶解性有机物(DOMs)混合后形成稳定纳米气泡(NB)团簇(半径约2纳米)的证据。值得注意的是,分子动力学模拟揭示了(不溶性)气体在水/DOM溶液中(随着DOMs摩尔分数的增加)经历溶解-纳米气泡-溶解的转变过程,这一现象在除极端极性情况外的大多数DOM类型中均有所观察。后一证据表明,含气体的NB团簇的形成可能是水中混合适量DOMs的普遍现象。
这种反复溶解行为可归因于DOMs的双重作用,即在低气体摩尔分数下促进和稳定NB团簇,并在超过NB形成阈值后生成气体的连续纳米结构。研究组独立的理论分析证实了水/DOM溶液中NB团簇的稳定性。与胶束形成类似,NB的形成提高了不溶性气体在水/DOM溶液中的溶解度。从基础溶液化学的角度来看,NB团簇的形成可以被视为一种纳米尺度的溶剂化机制,它与水中不溶性气体的传统分子溶剂化机制有显著不同。
附:英文原文
Title: Simulation Evidence of Nanobubble Clusters of Gas in Water: A Nanoscale Solvation Mechanism
Author: Jing Li, Hongguang Zhang, Zhenjiang Guo, Jian Jiang, Xianren Zhang, Dapeng Cao, Xiao Cheng Zeng
Issue&Volume: July 29, 2025
Abstract: Previous experiments have shown that when water is mixed with dissolvable organic molecules (DOMs), it can lead to insoluble gas-containing nanoclusters. However, molecular insights into the gas-containing nanoclusters are still lacking. Herein, we show the first large-scale simulation evidence of stable nanobubble (NB) clusters (~2 nm radius) of insoluble gas in water mixed with DOMs by using electrically neutral interface models. Notably, our molecular dynamics simulations demonstrate a dissolution–nanobubble–dissolution transition for the (insoluble) gas in the water/DOM solutions (as the mole fraction of DOMs is increased), a phenomenon observed across most DOM types excluding extreme polarity cases. The latter evidence suggests that the formation of gas-containing NB clusters is likely a generic phenomenon for water mixed with a modest amount of DOMs. The re-entrant dissolution behavior can be attributed to the dual roles of DOMs, i.e., for promoting and stabilizing NB clusters at low gas mole fraction and generating continuous nanostructures of the gas beyond the NB formation threshold. Our independent theoretical analysis confirms the stability of NB clusters in water/DOM solutions. Akin to micelle formation, NB formation results in increased solubility of insoluble gas in water/DOM solutions. From a fundamental solution chemistry perspective, the formation of NB clusters can be viewed as a nanoscale solvation mechanism that differs markedly from the conventional molecular solvation mechanism for the insoluble gas in water.
DOI: 10.1021/jacs.5c04560
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c04560
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
