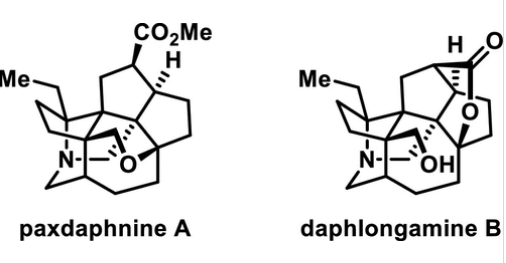
Paxdaphnine A(1)和daplongamine B(2)是两种七环水蚤类生物碱,每一种都含有8个连续的立体中心。
研究组通过仿生aza-Prins环化策略首次合成了这些外消旋形式的分子。Paxdaphnine B(3)被认为是1和2的生物成因前体。以可扩展的方式制备了3的五环类似物,并作为常见的中间体。该中间体的空间密集环戊烷部分是通过硝基-烯烃环加成反应构建的,其二醌基序的组装依赖于分子内的Pathemon-Khand反应。尽管3与HCHO的初始仿生aza-Prins反应结果不理想,但研究组利用3的N-氰甲基衍生物作为替代底物,与AgTFA作为启动子结合,挽救了1的仿生途径。基于类似的策略,一个aza-普林斯环化-内酯化级联,涉及不同的终止亲核试剂,被开发用于合成2。
附:英文原文
Title: Total Synthesis of Paxdaphnine A and Daphlongamine B via an Aza-Prins Cyclization Strategy
Author: Lu Ren, Zhaohong Lu, Dimin Wu, Mingchen Ma, Ang Li
Issue&Volume: July 23, 2025
Abstract: Paxdaphnine A (1) and daphlongamine B (2) are two heptacyclic Daphniphyllum alkaloids, each containing eight consecutive stereogenic centers. We achieved the first synthesis of these molecules in racemic form by using a biomimetic aza-Prins cyclization strategy. Paxdaphnine B (3), a putative biogenetic precursor of 1 and 2, was also obtained in the synthesis. A pentacyclic analogue of 3 was prepared in a scalable manner and served as a common intermediate. The sterically congested cyclopentane moiety of this intermediate was constructed via a nitrone–olefin cycloaddition reaction, and the assembly of its diquinane motif relied on an intramolecular Pauson–Khand reaction. Despite the unsatisfactory outcome of the initial biomimetic aza-Prins reaction of 3 with HCHO, we utilized the N-cyanomethyl derivative of 3 as an alternative substrate in combination with AgTFA as a promoter to rescue the biomimetic route to 1. On the basis of a similar strategy, an aza-Prins cyclization–lactonization cascade, involving a different terminating nucleophile, was developed for the synthesis of 2.
DOI: 10.1021/jacs.4c04779
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.4c04779
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
