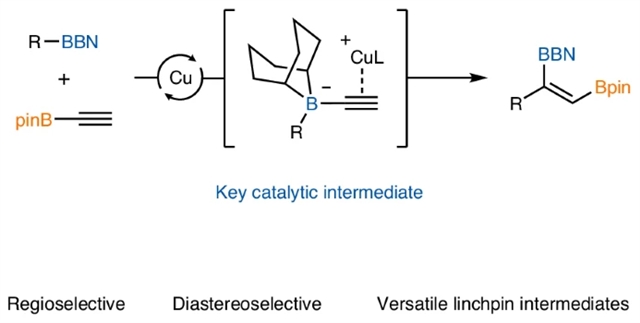
近日,美国华盛顿大学Lalic, Gojko团队研究了烷基硼烷催化C(sp2)同源化。这一研究成果发表在2025年7月24日出版的《自然-化学》杂志上。
有机硼化合物是有机合成中重要的中间体,常用于金属催化的交叉偶联反应。它们独特的反应性也允许修改它们的碳框架,同时保留有价值的硼基。传统上,这些同化反应仅限于通过C(sp3)插入C - B键形成烷基硼化合物。然而,最近在C(sp2)插入同源性方面的进展突出了这些反应在合成复杂烯烃方面的潜力,尽管目前在烯烃几何结构的范围和控制方面存在局限性。
研究组证明了催化C(sp2)插入同源性在区域和非对映选择性合成由简单烷基硼烷和炔基硼酯复杂的三取代二硼烯。他们的工作证明了广泛的反应范围和所得产物在高取代烯烃的模块化和立体选择性合成中的应用。此外,他们提供的证据支持一个独特的机制负责在反应中观察到良好的立体选择性。
附:英文原文
Title: Catalytic C(sp2) homologation of alkylboranes
Author: Gardner, Bradley W., Lalic, Gojko
Issue&Volume: 2025-07-24
Abstract: Organoboron compounds are important intermediates in organic synthesis, commonly used in metal-catalysed cross-coupling reactions. Their unique reactivity also allows modifications of their carbon framework with preservation of the valuable boryl group. Traditionally, these homologation reactions have been confined to the formation of alkyl boron compounds via C(sp3) insertion into a C–B bond. However, recent advancements in C(sp2)-insertive homologation highlight the potential of these reactions in synthesizing complex alkenes, despite current limitations in scope and control of the alkene geometry. Here we demonstrate a catalytic C(sp2)-insertive homologation for the regio- and diastereoselective synthesis of complex trisubstituted diborylalkenes from simple alkylboranes and alkynyl boronic esters. Our work demonstrates a broad reaction scope and application of the resulting products in modular and stereoselective synthesis of highly substituted alkenes. Furthermore, we provide evidence supporting a unique mechanism responsible for the excellent stereoselectivity observed in the reaction.
DOI: 10.1038/s41557-025-01854-4
Source: https://www.nature.com/articles/s41557-025-01854-4
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
