近日,华东理工大学?李其兆团队研究了具有可调芳构性和拓扑手性的交叉N-杂环共轭二聚体。2025年7月24日出版的《美国化学会杂志》发表了这项成果。
尽管通过低聚吡喃的氧化环化成功合成了独特的卟啉类化合物,但具有前所未有的拓扑结构,芳香性和手性的双环变体的开发仍然具有挑战性。
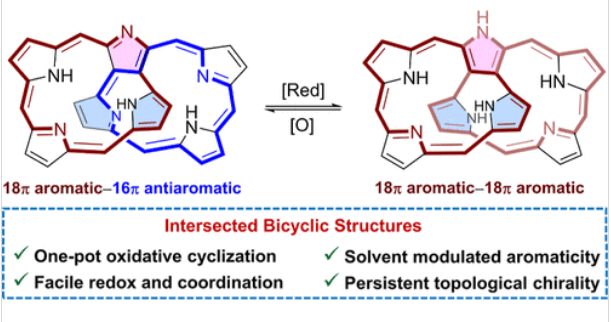
研究组报道了一类以七吡烷P7氧化环为基础的双环卟啉类化合物的合成。在此基础上,合成了一个非对称的交叉双环N-对映体1,其中两个末端吡咯α-C原子与中间吡咯环的β-C原子相连。二聚体1随后的还原通过两个氢原子的附着得到二聚体2。有趣的是,1和2分别表现出18π芳香- 16π反芳香和18π芳香- 18π芳香结构,并且2的芳香性可以通过溶剂极性和两性离子共振效应的贡献来有效调节。
此外,1和2都能螯合铜离子形成18π芳香-非芳香单铜(III)配合物1-Cu和非芳香-非芳香双铜配合物1-Cu2,吸收带边缘延伸到ca. 1800 nm。此外,这些化合物的独特结构使它们能够分离成一对对映体,其中(M,M)-2和(P,P)-2达到最大的不对称因子|gabs|为0.015。这些结果表明,长链低聚吡喃的氧化环化有望合成具有前所未有的拓扑结构、可调芳香性、光物理性质以及持久手性的新型双环卟啉类化合物。
附:英文原文
Title: Intersected Bicyclic N-Confused Corrole Dimers with Tunable Aromaticity and Topological Chirality
Author: Yanping Huang, Bin Zhu, Shijun Li, Glib Baryshnikov, Hailong Wang, Chengjie Li, Hans gren, Jianzhuang Jiang, Yongshu Xie, Qizhao Li
Issue&Volume: July 24, 2025
Abstract: Despite the successful syntheses of unique porphyrinoids by oxidative cyclization of oligopyrranes, the development of bicyclic variants with unprecedented topologies, aromaticity, and chirality remains challenging. Herein, we report the syntheses of a class of bicyclic porphyrinoids based on the oxidative cyclization of heptapyrrane P7. Thus, a nonsymmetrical intersected bicyclic N-confused corrole dimer 1 was synthesized, wherein two terminal pyrrolic α-C atoms are linked to the β-C atoms of the middle pyrrole ring. Subsequent reduction of dimer 1 afforded dimer 2 by the attachment of two hydrogen atoms. Interestingly, 1 and 2 exhibit 18π aromatic–16π antiaromatic and 18π aromatic–18π aromatic structures, respectively, and the aromaticity of 2 can be effectively modulated by solvent polarity with contribution of the zwitterionic resonance effect. Furthermore, both 1 and 2 can chelate copper ions to form 18π aromatic–nonaromatic mono-Cu(III) complex 1-Cu and nonaromatic–nonaromatic bis-Cu complex 1-Cu2, with the absorption band edge extended to ca. 1800 nm. In addition, the distinctive structures of these compounds enable their separation into a pair of enantiomers, with (M,M)-2 and (P,P)-2 achieving the largest dissymmetry factor |gabs| of 0.015. These results indicate that oxidative cyclization of long-chain oligopyrranes holds promise for the syntheses of novel bicyclic porphyrinoids with unprecedented topologies, tunable aromaticity, and photophysical properties as well as persistent chirality.
DOI: 10.1021/jacs.5c08284
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c08284
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000