近日,武汉大学闵昶团队研究了α,β-不饱和酮通过发散烷基胺插入的支架迁跃。该研究于2025年7月22日发表在《美国化学会志》上。
α、β-不饱和羰基化合物是有机合成中的关键中间体,广泛用于构建C-C和C-杂原子键的亲核加成反应中。伯胺是药物发现的重要组成部分,它们与α,β-不饱和羰基化合物反应产生药物,天然产物和生物活性分子的关键中间体。传统的方法通常强调1,2-或1,4-添加,针对外围修饰。同时,骨架氮插入反应主要对富电子烯烃或饱和酮位点有效,而α,β-不饱和酮的直接编辑通常仅限于有问题的施密特反应。
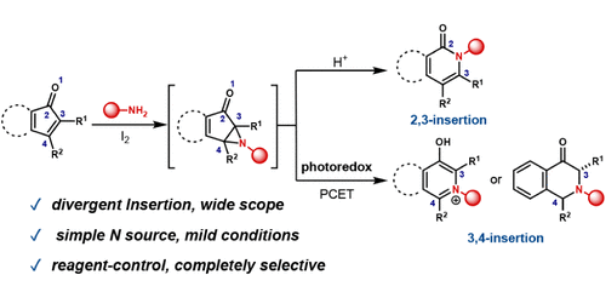
研究组通过2,3-和3,4-胺插入实现了α,β-不饱和酮的分化骨架编辑策略。这种方法有利于在同一底物的C-C键之间选择性地结合烷基胺。他们的试剂控制方案通过区域选择性叠氮中间体形成和靶向C-C键切割来调节插入位点,在一锅过程中产生大多数胺插入产物。重要的是,该策略为传统的施密特反应提供了差异和互补的区域选择性,允许从单一底物选择性合成各种吡啶基异构体,包括复杂的天然产物和候选药物。
附:英文原文
Title: Scaffold Hopping of α,β-Unsaturated Ketones via Divergent Alkyl Amine Insertion
Author: Huiguang Yu, Lili Xu, Luyao Li, Chang Min
Issue&Volume: July 22, 2025
Abstract: α,β-Unsaturated carbonyl compounds are pivotal intermediates in organic synthesis, widely utilized in nucleophilic addition reactions for constructing C–C and C–heteroatom bonds. Primary amines are essential building blocks in drug discovery, and their reactions with α,β-unsaturated carbonyl compounds yield key intermediates for pharmaceuticals, natural products, and bioactive molecules. Traditional methods often emphasize 1,2- or 1,4-additions, targeting peripheral modifications. Meanwhile, skeletal nitrogen-insertion reactions are primarily effective with electron-rich alkenes or saturated ketone sites, while the direct editing of α,β-unsaturated ketones is typically limited to problematic Schmidt reactions. In this study, we realize a divergent skeletal editing strategy for α,β-unsaturated ketones via 2,3- and 3,4-amine insertion. This approach facilitates the selective incorporation of alkyl amines between C–C bonds within the same substrate. Our reagent-controlled protocol enables tunable insertion sites through regioselective aziridine intermediate formation and targeted C–C bond cleavage, producing most amine insertion products in a one-pot process. Importantly, this strategy provides differential and complementary regioselectivity to traditional Schmidt reactions, allowing selective synthesis of various pyridyl isomers from a single substrate, including complex natural products and drug candidates.
DOI: 10.1021/jacs.5c06690
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c06690
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
