近日,美国布莱根妇女医院教授Soumya Raychaudhuri及其小组研制了在CRISPR编辑的单细胞中精确定义疾病变异效应。这一研究成果于2025年7月23日发表在国际顶尖学术期刊《自然》上。
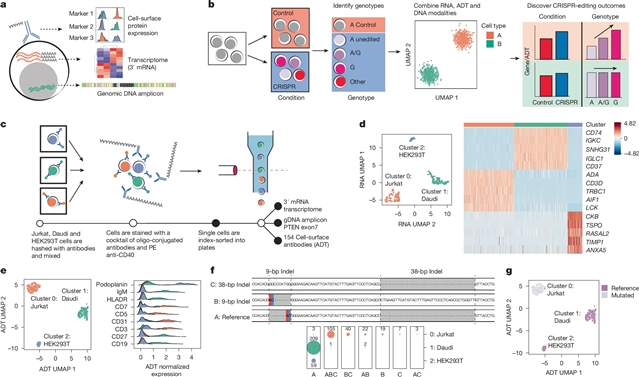
为了克服这些挑战,该团队提出了一种多组单细胞测序方法,可以直接识别基因组DNA编辑,分析转录组并测量细胞表面蛋白表达。该研究团队应用这种方法来研究基因破坏、调控区域缺失、非编码单核苷酸多态性等位基因和多重编辑的影响。小组确定了个体单核苷酸多态性的影响,包括IL2RA自身免疫变异在原代人T细胞中的状态特异性影响。包括DNA测序在内的多模态功能基因组单细胞测定使鉴定人类原代细胞的遗传变异成为可能,并弥补了在了解复杂人类疾病方面的重大差距。
研究人员表示,遗传学研究已经确定了单个疾病相关的非编码等位基因的需求,但遗传等位基因及其功能的鉴定仍然是一个关键瓶颈。CRISPR-Cas编辑使DNA靶向修饰能够引入和测试疾病等位基因。然而,单个细胞中低效的编辑、异质性的编辑结果以及由编辑和培养条件引起的非特异性转录变化的结合,限制了检测疾病等位基因功能后果的能力。
附:英文原文
Title: Precisely defining disease variant effects in CRISPR-edited single cells
Author: Baglaenko, Yuriy, Mu, Zepeng, Curtis, Michelle, Mire, Hafsa M., Jayanthi, Vidyashree, Al Suqri, Majd, Liu, Cassidy, Agnew, Ryan, Nathan, Aparna, Mah-Som, Annelise Yoo, Liu, David R., Newby, Gregory A., Raychaudhuri, Soumya
Issue&Volume: 2025-07-23
Abstract: Genetic studies have identified thousands of individual disease-associated non-coding alleles, but the identification of the causal alleles and their functions remains a critical bottleneck1. CRISPR–Cas editing has enabled targeted modification of DNA to introduce and test disease alleles. However, the combination of inefficient editing, heterogeneous editing outcomes in individual cells and nonspecific transcriptional changes caused by editing and culturing conditions limits the ability to detect the functional consequences of disease alleles2,3. To overcome these challenges, we present a multi-omic single-cell sequencing approach that directly identifies genomic DNA edits, assays the transcriptome and measures cell-surface protein expression. We apply this approach to investigate the effects of gene disruption, deletions in regulatory regions, non-coding single-nucleotide polymorphism alleles and multiplexed editing. We identify the effects of individual single-nucleotide polymorphisms, including the state-specific effects of an IL2RA autoimmune variant in primary human T cells. Multimodal functional genomic single-cell assays, including DNA sequencing, enable the identification of causal variation in primary human cells and bridge a crucial gap in our understanding of complex human diseases.
DOI: 10.1038/s41586-025-09313-3
Source: https://www.nature.com/articles/s41586-025-09313-3
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
