2025年7月21日出版的《美国化学会志》发表了香港理工大学
金属β-内酰胺酶作为抗菌素耐药性危机的强大对手的出现源于其无与伦比的水解β-内酰胺抗生素的能力。
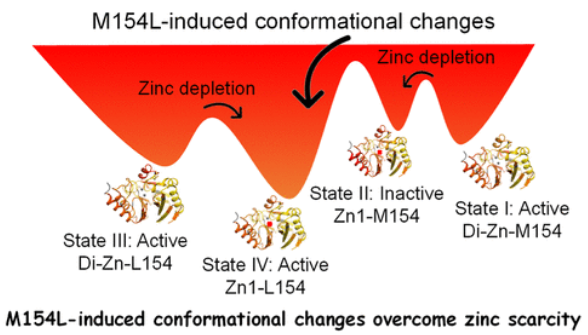
研究组通过构象动力学研究揭示了新德里金属β内酰胺酶(NDM)变异的进化策略。使用氢/氘交换质谱(HDX-MS)分别绘制了NDM在无配体状态和与抑制剂l-卡托普利、d-卡托普利、依布selen和曲霉酰胺A (AMA)结合状态下的构象图。至关重要的是,他们的发现揭示了类似的变构指纹对应于不同的抑制机制;也就是说,抑制作用在α3-L8 -β8区域诱发了明显的动态扰动,这是一个以前未被充分表征的区域。引人注目的是,临床上普遍存在的M154L突变重塑了该区域的构象灵活性,在不改变l/d-卡托普利结合动力学的情况下放大了抑制剂特异性构象反应。
这项研究表明,在AMA和ebselen缺乏锌的情况下,单个突变如何对抗生素耐药性进化至关重要,α3-L8区域和几个活性位点环( ASL)区域的HDX模式受到更多保护。他们的研究结果建立了三个关键的进展:(1)α3-L8是一个控制构象适应性的隐变变区;(2)证明了单个突变M154L重新连接远程动态通信;(3)提出了构象引导抑制剂设计作为抗NDM的可行策略。总的来说,这项工作揭示了一个新的视角──抗性突变不仅是化学优化剂,而且是利用固有蛋白质可塑性的变构调节剂。这些发现将α3-L8区域定位为开发新型抑制剂的引人注目的靶点,为对抗下一个抗微生物药物耐药性前沿提供了蓝图。
附:英文原文
Title: Inhibitor-Dependent Tolerance of New Delhi Metallo-β-Lactamase Driven by Single Mutation-Induced Conformational Changes
Author: Liwen Huang, Tsz-Fung Wong, Qipeng Cheng, Pui-Kin So, Ming Liu, Xuechen Li, Sheng Chen, Zhong-Ping Yao
Issue&Volume: July 21, 2025
Abstract: The emergence of metallo-β-lactamases as formidable adversaries in the antimicrobial resistance crisis stems from their unparalleled capacity to hydrolyze β-lactam antibiotics. This study deciphers the evolutionary strategy of New Delhi metallo-β-lactamase (NDM) variants through studies of conformational dynamics. We employ hydrogen/deuterium exchange mass spectrometry (HDX-MS) to map conformational landscapes of NDM in the ligand-free state and in the bound states with inhibitors l-captopril, d-captopril, ebselen, and aspergillomarasmine A (AMA), respectively. Crucially, our findings reveal similar allosteric fingerprints corresponding to different inhibition mechanisms; that is, inhibition induces pronounced dynamic perturbations in the α3–L8–β8 region─a previously under-characterized region. Strikingly, the clinically prevalent M154L mutation in this region reshapes conformational flexibility, amplifying inhibitor-specific conformational responses without altering the l/d-captopril binding dynamics. This study demonstrates how a single mutation can be critical for antibiotic resistance evolution where zinc is scarce in the presence of AMA and ebselen, as indicated by more protected HDX patterns of the α3–L8 region and several active-site loop (ASL) regions. Our results establish three key advances: (1) identification of α3–L8 as a cryptic allosteric region governing conformational adaptability, (2) demonstration of a single mutation M154L rewiring long-range dynamic communication, and (3) proposal of conformation-guided inhibitor design as a viable strategy against NDM. Overall, this work unveils a novel perspective─resistance mutations function not merely as chemical optimizers but as allosteric modulators that exploit inherent protein plasticity. These insights position the α3–L8 region as a compelling target for developing novel inhibitors, providing a blueprint for combating the next frontier of antimicrobial resistance.
DOI: 10.1021/jacs.5c05669
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c05669
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
