德国曼斯特大学Gilmour, Ryan团队近日实现了利用具有优越性能的铝-Salen催化剂,实现能量转移驱动的对映选择性光环化反应。这一研究成果于2025年7月17日发表在《自然-化学》杂志上。
手性催化剂可以通过不同的基态激活模式与多种底物结合,以高保真度提供富含对映体的产物,这通常被称为“特权”。在激发态过程中实现普遍性仍然具有挑战性,并且正在努力确定特殊的手性光催化剂。铝-盐配合物由于其良好的光物理性质而成为新兴的竞争者。
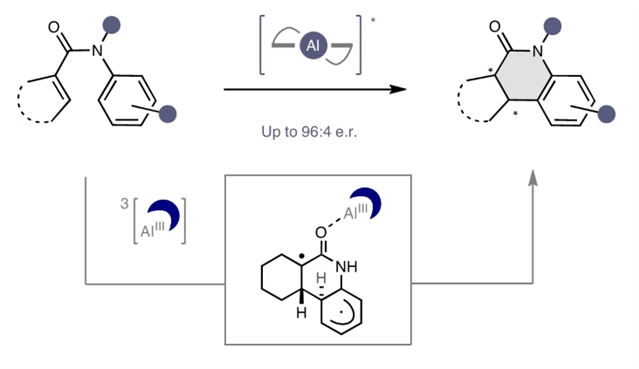
研究组报道了一种对映选择性能量转移(EnT)催化丙烯酰胺的光环化的发展,以扩大Al-salen光催化剂的激活范围。这种方法允许反应性和对映体选择性同时由一个廉价的,商业手性Al-salen配合物在λ = 400 nm照射下调节。不同的环状产物可以锻造具有高水平的对映体选择性(高达96:4 e.r)。在激化态激活模式中建立这种二分法有助于巩固手性Al-salen配合物在对映选择性光催化中的特殊地位,并补充它们在基态机制中的普遍存在。
附:英文原文
Title: Energy transfer-enabled enantioselective photocyclization using a privileged Al–salen catalyst
Author: Soika, Julia, Onneken, Carina, Wiegmann, Thorben, Stnkel, Timo, Morack, Tobias, Lindfeld, Leander, Hebenbrock, Marian, Mck-Lichtenfeld, Christian, Neugebauer, Johannes, Gilmour, Ryan
Issue&Volume: 2025-07-17
Abstract: Chiral catalysts that can engage multiple substrates, via distinct ground-state activation modes, to deliver enantioenriched products with high levels of fidelity are often described as ‘privileged’. Achieving generality in excited-state processes remains challenging, and efforts to identify privileged chiral photocatalysts are being intensively pursued. Aluminium–salen complexes are emergent contenders on account of their well-defined photophysical properties. Here we report the development of an enantioselective energy transfer (EnT) catalysis-enabled photocyclization of acrylanilides to expand the activation repertoire of Al–salen photocatalysts. This approach allows reactivity and enantioselectivity to be simultaneously regulated by an inexpensive, commercial chiral Al–salen complex upon irradiation at λ=400nm. Diverse cyclic products can be forged with high levels of enantioselectivity (up to 96:4 e.r.). Establishing this dichotomy in excited-state activation modes serves to consolidate the privileged status of chiral Al–salen complexes in enantioselective photocatalysis and to complement their ubiquity in ground-state regimes.
DOI: 10.1038/s41557-025-01857-1
Source: https://www.nature.com/articles/s41557-025-01857-1
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
