近日,英国布里斯托大学Beatrice S. L. Collins团队研究了酶控制下氧化还原动力的自主定向C-C键旋转。2025年7月16日,《自然》杂志发表了这一成果。
活的生物系统依赖于化学反应网络的持续运行。这些网络保持着不平衡状态,其中化学能不断地转化为受控的机械功和运动。非平衡反应网络也使人工自主操作分子机器的设计和成功开发成为可能,在这种机器中,由成对的形式(但非微观)逆反应途径组成的网络在分子水平上驱动受控运动。
在生物系统中,多种反应途径的同时运行是由酶及其辅助因子的化学选择性实现的,自然界的耗散反应网络包括几种反应。相比之下,用于开发化学反应网络以追求人工分子机器的反应性仅限于单一反应类型。只有少数合成体系表现出化学燃料驱动的连续分子水平运动,并且都利用同一类酰化水解反应。
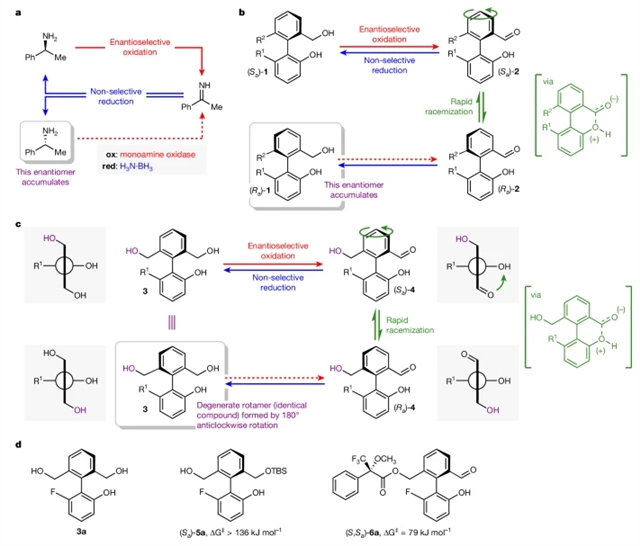
研究组表明,在一个结构简单的基于非手性联苯的合成分子马达中,一个由同步氧化和还原途径组成的氧化还原反应网络可以驱动化学燃料的C-C键的连续自主单向运动。氧化剂和还原剂作为燃料的组合主题以及马达的方向性都通过利用酶催化固有的反应性的对映选择性和功能分离来实现。
附:英文原文
Title: Redox-powered autonomous directional C–C bond rotation under enzyme control
Author: Berreur, Jordan, Watts, Olivia F. B., Bulless, Theo H. N., ODonoghue, Nicholas T., Del Olmo, Marc, Winter, Ashley J., Clayden, Jonathan, Collins, Beatrice S. L.
Issue&Volume: 2025-07-16
Abstract: Living biological systems rely on the continuous operation of chemical reaction networks. These networks sustain out-of-equilibrium regimes in which chemical energy is continually converted into controlled mechanical work and motion1,2,3. Out-of-equilibrium reaction networks have also enabled the design and successful development of artificial autonomously operating molecular machines4,5, in which networks comprising pairs of formally—but non-microscopically—reverse reaction pathways drive controlled motion at the molecular level. In biological systems, the concurrent operation of several reaction pathways is enabled by the chemoselectivity of enzymes and their cofactors, and nature’s dissipative reaction networks involve several classes of reactions. By contrast, the reactivity that has been harnessed to develop chemical reaction networks in pursuit of artificial molecular machines is limited to a single reaction type. Only a small number of synthetic systems exhibit chemically fuelled continuous controlled molecular-level motion6,7,8 and all exploit the same class of acylation–hydrolysis reaction. Here we show that a redox reaction network, comprising concurrent oxidation and reduction pathways, can drive chemically fuelled continuous autonomous unidirectional motion about a C–C bond in a structurally simple synthetic molecular motor based on an achiral biphenyl. The combined use of an oxidant and reductant as fuels and the directionality of the motor are both enabled by exploiting the enantioselectivity and functional separation of reactivity inherent to enzyme catalysis.
DOI: 10.1038/s41586-025-09291-6
Source: https://www.nature.com/articles/s41586-025-09291-6
官方网址:http://www.nature.com/
