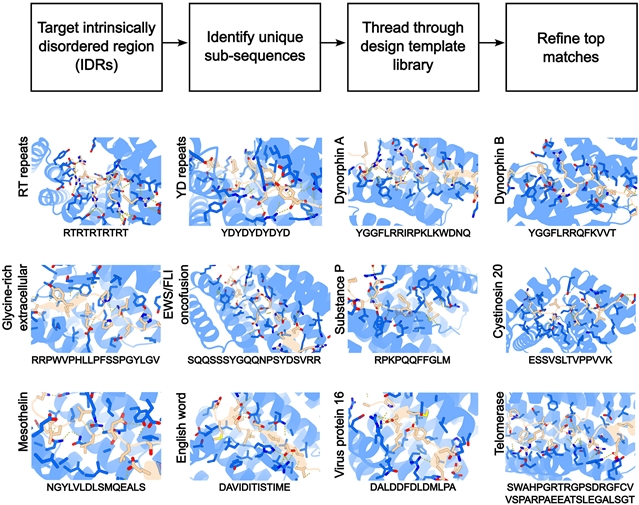
课题组描述了一种设计蛋白质的一般方法,这种蛋白质以不同的延伸构象结合内在无序的蛋白质区域,侧链适合于互补的结合口袋。该课题组人员将这种方法用于39种高度多样化的非结构化靶标(包括极性靶标)的设计,并在34种情况下获得了100皮摩尔到100纳摩尔的亲和设计,每个靶标测试了22种设计。这些设计在细胞中起作用,并作为检测试剂,在所有结合实验中对其预期目标具有特异性。他们的方法是朝着内在无序的蛋白质和肽识别问题的一般解决方案迈出的重要一步。
研究人员表示,内在无序的蛋白质和肽在生物学中发挥着关键作用,但缺乏明确的结构和序列和构象偏好的高度可变性使得靶向此类系统具有挑战性。
附:英文原文
Title: Design of intrinsically disordered region binding proteins
Author: Kejia Wu, Hanlun Jiang, Derrick R. Hicks, Caixuan Liu, Edin Muratspahi, Theresa A. Ramelot, Yuexuan Liu, Kerrie McNally, Sebastian Kenny, Andrei Mihut, Amit Gaur, Brian Coventry, Wei Chen, Asim K. Bera, Alex Kang, Stacey Gerben, Mila Ya-Lan Lamb, Analisa Murray, Xinting Li, Madison A. Kennedy, Wei Yang, Zihao Song, Gudrun Schober, Stuart M. Brierley, John O’Neill, Michael H. Gelb, Gaetano T. Montelione, Emmanuel Derivery, David Baker
Issue&Volume: 2025-07-17
Abstract: Intrinsically disordered proteins and peptides play key roles in biology, but a lack of defined structures and high variability in sequence and conformational preferences have made targeting such systems challenging. We describe a general approach for designing proteins that bind intrinsically disordered protein regions in diverse extended conformations with side chains fitting into complementary binding pockets. We used the approach to design binders for 39 highly diverse unstructured targets, including polar targets, and obtained designs with 100-picomolar to 100-nanomolar affinities in 34 cases, testing ~22 designs per target. The designs function in cells and as detection reagents and are specific for their intended targets in all-by-all binding experiments. Our approach is a major step toward a general solution to the intrinsically disordered protein and peptide recognition problem.
DOI: adr8063
Source: https://www.science.org/doi/10.1126/science.adr8063
