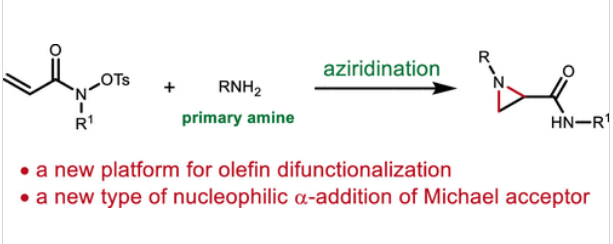
中山大学刘文博团队近日实现了亲核的α-和β-添加物使共轭羟基酸盐氧化还原-中性氮化。相关论文于2025年7月15日发表在《美国化学会志》上。
在氧化条件下,以非保护伯胺为氮供体的烯烃叠氮化反应是非常理想的,但由于非保护胺与氧化剂的不相容,这带来了挑战。
为了解决这个问题,研究组提出了一种利用内部氧化剂的策略。这种方法使亲核的α-和β-胺添加到共轭羟基酸盐,实现从简单和安全的构建块组装复杂的叠氮嘧啶。受益于这种内部氧化剂的概念,富电子苯胺被直接用于接触N-芳基叠氮嘧啶。除了叠氮化外,当仲胺被主题化时,也可以实现烯烃1,2-二胺化。机制研究表明,α-内酰胺中间体参与了这种叠氮化,使氧化还原转位成为可能。在更广泛的背景下,这种转化代表了一种新型的亲核α-加成,与更常见的β-加成(Michael加成)相比,这是罕见的。
附:英文原文
Title: Nucleophilic α- and β-Additions Enable Redox-Neutral Aziridination of Conjugated Hydroxamates
Author: Rui Wang, Quanbin Jiang, Long Jiang, Wenbo H. Liu
Issue&Volume: July 15, 2025
Abstract: Aziridination of olefins using nonprotected primary amines as nitrogen donors under oxidative conditions is highly desirable but poses challenges due to the incompatibility of nonprotected amines with oxidants. To address this issue, a strategy to leverage an internal oxidant is proposed. This method enables nucleophilic α- and β-additions of amines to conjugated hydroxamates, allowing for the assembly of complex aziridines from simple and safe building blocks. Benefiting from this internal oxidant concept, electron-rich anilines are directly employed to access N-aryl aziridines. Beyond aziridination, olefin 1,2-diamination can also be achieved when secondary amines are used. Mechanistic studies suggest that an α-lactam intermediate is involved in this aziridination to enable the redox-transposition. In a broader context, this transformation represents a novel type of nucleophilic α-addition, which is rare compared to the more common β-addition (the Michael addition).
DOI: 10.1021/jacs.5c04286
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c04286
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
