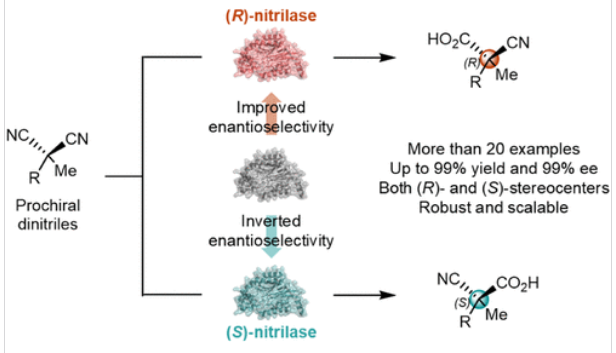
含一对对映官能团的全取代碳的去对称化是合成四元立体中心的实用方法。前手性α,α-二取代丙二腈由于其制备简单,功能取代基的可编程引入以及所得到的对映体富集腈的高合成价值而受到特别关注。然而,这些丙二腈的不对称催化转化为全碳季立体中心是一个重大的挑战。
研究组提出了一种腈酶(SsNIT)催化的α,α-二取代丙二腈的去对称水解,从而生产出含有无环全碳季位中心的对映富集α-氰羧酸,产率高(高达99%),对映选择性高(高达99% ee)。通过基于福克主题的理性化多轮循环定点突变技术(FRISM)对酶的底物进行微调和重塑,研究组成功地设计了一对对映互补腈酶(M1和M3),从而提供了一种不同的合成途径来组装R和S构型的四元立体中心。
通过以全细胞生物催化剂为主题的克级反应进一步验证了该策略的合成稳定性和可扩展性,随后衍生成各种富含对映体的非规范氨基酸衍生物,并带有季立体中心。这项研究强调了生物催化在通过合理的酶工程促进高效合成季中心及其对映体方面的潜力。
附:英文原文
Title: Enantiodivergent Access to Acyclic Quaternary Stereocenters by Nitrilase-Catalyzed Desymmetrizing Hydrolysis of Malononitriles
Author: Yong-Ze Xie, Dong-Sheng Yang, Li-Gang Bai, Jin-Wei Yang, Bo Gu, Meng-Ting Liu, Qi-Yue Wang, Wang Wang, Wen-Bo Liu, Heng Song
Issue&Volume: July 15, 2025
Abstract: The desymmetrization of fully substituted carbons bearing a pair of enantiotopic functional groups represents a pragmatic approach to the synthesis of quaternary stereocenters. Prochiral α,α-disubstituted malononitriles are of particular interest due to the simplicity of their preparation, the programmable introduction of functional substituents, and the high synthetic value of the resulting enantioenriched nitriles. Nevertheless, the asymmetric catalytic transformation of these malononitriles to all-carbon quaternary stereocenters represents a significant challenge. Here, we present a nitrilase (SsNIT)-catalyzed desymmetrizing hydrolysis of α,α-disubstituted malononitriles, resulting in the production of enantioenriched α-cyanocarboxylic acids containing acyclic all-carbon quaternary stereocenters in good yields (up to 99% yield) with excellent enantioselectivities (up to 99% ee). By fine-tuning and reshaping the substrate pockets of the enzyme through Focused Rational Iterative Site-directed Mutagenesis (FRISM), we successfully engineered a pair of enantiocomplementary nitrilases (M1 and M3), thereby providing a divergent synthetic route to assemble both R and S configurations of the quaternary stereocenters. The synthetic robustness and scalability of this strategy were further validated through gram-scale reactions using whole-cell biocatalysts, followed by derivatization into diverse enantioenriched noncanonical amino acid derivatives bearing quaternary stereocenters. This study highlights the potential of biocatalysis in facilitating the efficient synthesis of quaternary centers and their enantiomers through the rational engineering of enzymes.
DOI: 10.1021/jacs.5c09258
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c09258
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
