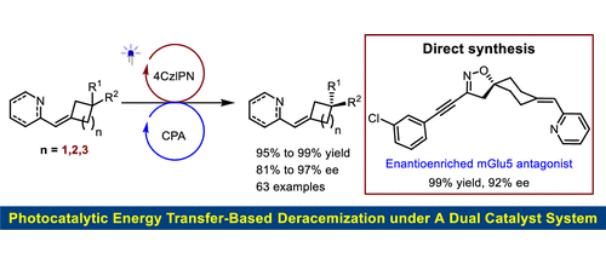
近日,河南师范大学江智勇团队研究了双催化剂体系下基于三态能量转移的轴向手性烯烃离消旋反应。相关论文发表在2025年7月14日出版的《美国化学会志》上。
光化学去中心化是不对称合成中一种高效的方法。因此,一些关键的手性光敏剂已经开发参与能量转移(EnT)为基础的机制。然而,催化剂类型的有限多样性和光敏剂部分对对映体选择性的明显空间效应是一个固有的挑战,从而极大地限制了底物的范围。在这种情况下,由于独立选择两种催化剂的灵活性,探索双催化剂体系的可行性成为一项关键任务。值得注意的是,即使在没有手性催化剂的情况下,对映体富集产物的固有外消旋化也是影响对映体富集效率的一个重要障碍。
尽管存在这些挑战,研究组已经成功地实现了这一目标,提供了机器人概念验证。因此,在由可见光介导的手性磷酸(CPA)和4CzIPN组成的双催化剂体系下,可以合成一系列有价值的轴向手性氮杂芳基环烷烃,并具有优异的产率和对映选择性。底物的范围非常广泛,包括广泛的环己烷、环戊烷和环丁烷,它们被各种各样的氮杂芳烃取代,并具有不同的立体中心构型。值得注意的是,这包括螺旋和全碳的四元立体中心,它们都表现出特殊的相容性。更重要的是,许多生物活性分子,如关键的mGlu5拮抗剂,可以直接合成,精度高,进一步突出了该工作的意义。
附:英文原文
Title: Triplet Energy Transfer-Based Deracemization of Axially Chiral Alkenes Enabled by a Dual Catalyst System
Author: Guangkuo Zeng, Wenshuo Shi, Zhuoxi Wang, Xiaowei Zhao, Yanli Yin, Zhiyong Jiang
Issue&Volume: July 14, 2025
Abstract: Photochemical deracemization has been recognized as a highly efficient strategy in asymmetric synthesis. Consequently, several pivotal chiral photosensitizers have been developed to participate in energy transfer (EnT)-based mechanisms. Nevertheless, the limited diversity of catalyst types and the pronounced spatial effects of photosensitizer moieties on enantioselectivity present an inherent challenge, thereby significantly restricting the substrate scope. In this context, exploring the feasibility of dual-catalyst systems becomes a critical task due to the flexibility of independently selecting two catalysts. Notably, the intrinsic racemization of enantioenriched products, even in the absence of chiral catalysts, represents a substantial obstacle that considerably impacts the efficiency of enantiomer enrichment. Despite these challenges, we have successfully achieved this objective, providing robust proof-of-concept validation. As a result, under a dual-catalyst system comprising a chiral phosphoric acid (CPA) and 4CzIPN mediated by visible light, a broad range of valuable axially chiral azaarylidene cycloalkanes can be synthesized with exceptional yields and enantioselectivities. The scope of the substrates is remarkably extensive, including a wide range of cyclohexanes, cyclopentanes, and cyclobutanes substituted with various azaarenes and featuring diverse stereocenter configurations. Notably, this encompasses spiro- and all-carbon quaternary stereogenic centers, all of which exhibit exceptional compatibility. More importantly, numerous bioactive molecules, such as a key mGlu5 antagonist, can be directly synthesized with high precision, further highlighting the significance of this work.
DOI: 10.1021/jacs.5c10123
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c10123
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
