近日,中国科学院上海光学精密机械研究所杨帆团队研究了峰值功率为780 nm脉冲激光系统的受激布里渊散射显微镜。相关论文于2025年7月10日发表在《自然—光子学》杂志上。
受激布里渊散射显微镜使全光学、非接触、高空间分辨率的机械成像具有高特异性。准连续波受激布里渊散射的最新进展大大降低了所需的激光功率。然而,像素停留时间仍限制在20 ms,在不损害分辨率、精度或特异性的情况下进一步降低是具有挑战性的。
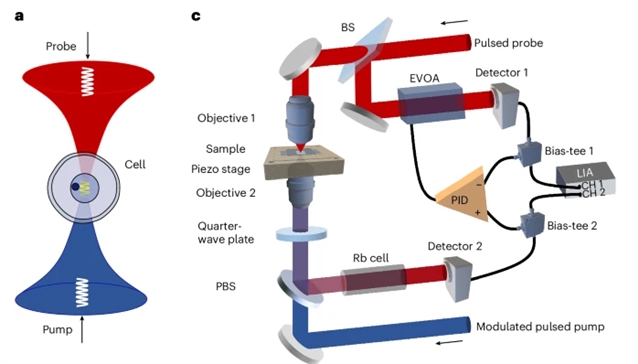
研究组通过开发一种工作在780nm的倍频脉冲光纤激光系统来解决这一局限性,该系统可提供267W的峰值功率。为了减轻放大光纤激光器固有的高强度噪声,他们实现了一种基于自动平衡检测的高性能降噪系统。因此,该研究组实现了像素停留时间短至200μs的全光谱捕获,比以前的受激布里渊散射实现快两个数量级,并保持平均功率为30mW。
研究组展示了活单细胞、类器官、斑马鱼幼虫和卵巢卵泡的亚细胞细节的高速、高特异性和高灵敏度布里渊成像。此外,他们还捕获了秀丽隐杆线虫早期胚胎发育过程中快速细胞分裂的体内生物力学动力学。
附:英文原文
Title: Stimulated Brillouin scattering microscopy with a high-peak-power 780-nm pulsed laser system
Author: Qi, Yun, Yao, Shuai, Du, Zi-Xuan, Zhang, Jin-Rui, Zhou, Cuiyun, Fu, Xiaohu, Li, Huan, Mi, Ting, Chen, Yu-Han, Wang, Yu-Fan, Luo, Yun, He, Xuyan, Nan, Jing, Zhang, Yanjie, Sun, Lin, Xia, Peng, Cai, Shi-Qing, Du, Jiu-Lin, Xie, Jingjing, Chen, Wei-Biao, Yang, Fan
Issue&Volume: 2025-07-10
Abstract: Stimulated Brillouin scattering microscopy enables all-optical, non-contact, high-spatial-resolution mechanical imaging with high specificity. Recent advances in quasi-continuous-wave stimulated Brillouin scattering have substantially reduced the required laser power. However, the pixel dwell time remains limited to 20ms, and further reductions without compromising resolution, precision or specificity are challenging. Here we address this limitation by developing a frequency-doubled pulsed fibre laser system operating at 780nm, delivering a peak power of 267W. To mitigate the high-intensity noise intrinsic to amplified fibre lasers, we implement a high-performance noise cancellation system based on auto-balanced detection. As a result, we achieve a pixel dwell time as short as 200μs for full spectral acquisition, which is two orders of magnitude faster than previous stimulated Brillouin scattering implementations, and maintain an average power of 30mW. We demonstrate high-speed, high-specificity and high-sensitivity Brillouin imaging of live single cells, organoids, zebrafish larvae and ovarian follicles with subcellular details. Furthermore, we capture the in vivo biomechanical dynamics of rapid cell divisions during the early embryo development of Caenorhabditis elegans in a living worm.
DOI: 10.1038/s41566-025-01697-y
Source: https://www.nature.com/articles/s41566-025-01697-y
