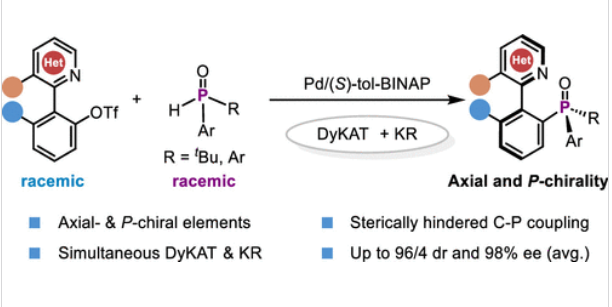
上海交通大学刘家旺团队近日研究了通过同时进行动态动力学不对称转化和动力学拆分,钯催化轴向和对手性膦氧化物的对映体和对映体选择性C-P偶联。相关论文于2025年6月30日发表在《美国化学会志》上。
具有轴向和P-手性的阻转异构体的催化不对称合成非常有吸引力,但仍然是一个重大的合成挑战。
研究组报道了一种Pd催化的非对映和对映选择性C-P偶联反应,该反应通过异联芳基的动态动力学不对称转化和非对称仲氧化膦的动力学拆分相结合,实现了P-对映异联芳基非对映选择性构建。该方案具有广泛的底物范围和优异的官能团耐受性,以高产率和优异的非对映选择性提供各种轴向和P-手性氧化膦,具有近乎完美的对映选择性。该方法的合成实用性进一步通过多手性路易斯碱催化剂的简单制备和手性P,N-配体如QUINAP的不对称合成得到了证明。
附:英文原文
Title: Diastereo- and Enantioselective Pd-Catalyzed C–P Coupling for Axially and P-Chiral Phosphine Oxides via Simultaneous Dynamic Kinetic Asymmetric Transformation and Kinetic Resolution
Author: Shen Gao, Lei Su, Jiawang Liu
Issue&Volume: June 30, 2025
Abstract: The catalytic asymmetric synthesis of atropisomers bearing axial and P-chirality is highly appealing yet remains a significant synthetic challenge. Herein, we report a Pd-catalyzed diastereo- and enantioselective C–P coupling reaction that enables the atroposelective construction of P-stereogenic heterobiaryls via the combination of dynamic kinetic asymmetric transformation of heterobiaryls and kinetic resolution of nonsymmetric secondary phosphine oxides. This protocol exhibits a broad substrate scope with excellent functional group tolerance, delivering a diverse array of axial and P-chiral phosphine oxides in high yields and excellent diastereoselectivities with near-perfect enantioselectivities. The synthetic utility of this method is further demonstrated by the facile preparation of multichiral Lewis base catalysts and the asymmetric synthesis of chiral P,N-ligands such as QUINAP.
DOI: 10.1021/jacs.5c07049
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c07049
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000