厦门大学姜涛团队近日实现了利用多面循环构造形成肽组合。这一研究成果发表在2025年6月30日出版的《美国化学会志》上。
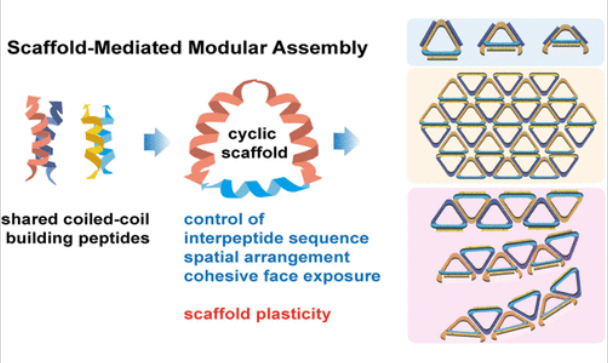
构建不同的生物大分子组装体通常需要靶向选择和构建模块的工程设计,同时优化组装条件。挑战在于在相同条件下使用简单的共享模块实现不同的形态结果,这是自然系统的一个标志,在合成方法中仍然难以捉摸。研究组提出了一种基于分子支架的策略,指导同一组肽在多个维度上共组装成各种纳米结构。他们创建了三乙酰化环状支架,在共组装之前操纵两对二聚体卷曲线圈肽。
这些支架具有可寻址和正交的模块,可以控制其粘合面的暴露,指导纳米三角形、原纤维和层状组件的形成。通过调整决定支架几何形状的肽间排列,研究组构建了具有可调曲率的非直线原纤维,这在以前很少见。值得注意的是,这些支架在使内聚面的大小和方向适应不同的组装形态方面表现出可塑性。所得纳米结构与设计和模拟结果一致,证明了这种方法的可靠性和可预测性。多方面的环状支架弥合了构建肽和组装之间的智力和物理差距,有望赋予各种现有的组装系统高度的可调性和通用性。
附:英文原文
Title: Shaping Peptide Assemblies Using Multifaceted Cyclic Tectons
Author: Chenru Wang, Dexin Lu, Jiakang Li, Linyuan Chen, Xiaobing Zuo, Chuanliu Wu, Xian-Kui Wei, Binju Wang, Yun-Bao Jiang, Tao Jiang
Issue&Volume: June 30, 2025
Abstract: Constructing distinct biomacromolecular assemblies typically necessitates target-specific selection and engineering of building blocks alongside optimization of assembly conditions. The challenge lies in achieving diverse morphological outcomes using simple, shared modules under identical conditions, a hallmark of natural systems that remains elusive in synthetic approaches. Here, we present a molecular scaffold-based strategy to instruct the coassembly of the same set of peptides into a variety of nanostructures across multiple dimensions. We create trifaceted cyclic scaffolds to manipulate two pairs of dimeric coiled-coil peptides prior to coassembly. These scaffolds, with addressable and orthogonal modules, allow controlled exposure of their cohesive faces, directing the formation of nanotriangles and fibrillar and lamellar assemblies. By tuning interpeptide arrangements that dictate scaffold geometry, we construct nonstraight fibrils with tunable curvature, which are rarely observed before. Notably, these scaffolds exhibit plasticity in adapting the sizes and orientations of cohesive faces to different assembly morphologies. The resultant nanostructures are consistent with the design and simulation results, demonstrating the reliability and predictability of this approach. Multifaceted cyclic scaffolds bridge the intellectual and physical gaps between building peptides and assemblies, holding promise for endowing various existing assembly systems with high tunability and versatility.
DOI: 10.1021/jacs.5c04788
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c04788
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
