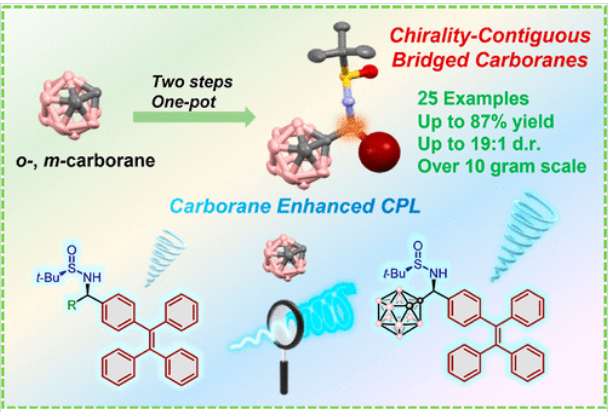
近日,西安交通大学焦佼团队研究了手性连续桥联碳硼烷:圆极化发光的协同可扩展合成和放大。2025年6月30日出版的《美国化学会杂志》发表了这项成果。
二十面体碳硼烷因其独特的三维芳香结构和独特的电子性能而成为光电功能材料的有前景的结构基序。然而,由于缺乏高效和可扩展的不对称合成以及有限的圆偏振发光(CPL)研究,它们的应用仍然受到限制。研究组开发了一种高度立体选择性和直接不对称的手性碳硼烷分子合成方法。该方法采用现成的起始材料,通过两步一锅法精确构建光学纯手性连续桥接碳硼烷衍生物,实现了10克规模的可扩展合成,而无需手性HPLC分辨率。在有机共轭发色团上引入手性碳硼烷取代基后,获得了一系列性能优异的CPL活性分子。它们的发光不对称因子(glum)达到了10-3的水平,量子产率(ΦPL)达到了0.70。
此外,研究组已经观察到,将碳硼烷引入手性桥接结构显著提高了发光化合物的ΦPL和glum值。这两个参数的同时增强几乎没有记录。应用其中一种手性发射化合物,实现了对水相中几种游离氨基酸的良好对映选择性区分。该研究为多种手性碳硼烷衍生物的立体选择性大规模合成提供了一条有效可行的合成路线。在光物理度量和手性识别应用方面所展示的增强突出了这些混合系统的双重功能,为在聚合物科学、药物化学和光电子领域开发手性材料提供了战略见解。
附:英文原文
Title: Chirality-Contiguous Bridged Carboranes: Synergistic Scalable Synthesis and Amplification of Circularly Polarized Luminescence
Author: Longfei Nan, Pei He, Tingwei Zhang, Yiran Dong, Chao Feng, Tong He, Pengfei Li, Yanfeng Zhang, Yong Nie, Jiao Jiao
Issue&Volume: June 30, 2025
Abstract: Icosahedral carboranes are promising structural motifs for optoelectronic functional materials owing to their unique three-dimensional aromatic architectures and distinctive electronic properties. However, their applications remain restricted by the absence of efficient and scalable asymmetric synthesis and limited circularly polarized luminescence (CPL) studies. Herein, we developed a highly stereoselective and straightforward asymmetric synthesis for chiral carborane molecules. This method employs readily available starting materials and enables the precise construction of optically pure chirality-contiguous bridged carborane derivatives via a two-step, one-pot protocol, achieving scalable synthesis on a 10 g-scale without chiral HPLC resolution. After chiral carborane substituents were introduced onto the organic conjugated chromophores, a series of CPL-active molecules with excellent performance were obtained. Their luminescence dissymmetry factors (glum) reached up to 10–3 level, and the quantum yields (ΦPL) achieved up to 0.70. Furthermore, it has been observed that the introduction of carborane into the chiral bridging structure significantly enhances the ΦPL and glum values of the luminescent compounds. Such concurrent enhancement of both parameters is scarcely documented. Applying one of the chiral emissive compounds, a good enantioselective discrimination of several free amino acids in the aqueous phase has been achieved. This study provides an effective and feasible synthetic route for the stereoselective large-scale synthesis of diverse chiral carborane derivatives. The demonstrated enhancements in both photophysical metrics and chiral recognition applications highlight the dual functionality of these hybrid systems, providing strategic insights for developing chiral materials in polymer science, pharmaceutical chemistry, and optoelectronics.
DOI: 10.1021/jacs.5c03381
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c03381
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
