美国加州大学David S. Eisenberg研究组取得一项新突破。他们开发出短肽如何在阿尔茨海默病中分解tau原纤维。2025年7月9日出版的《自然》杂志发表了这项成果。
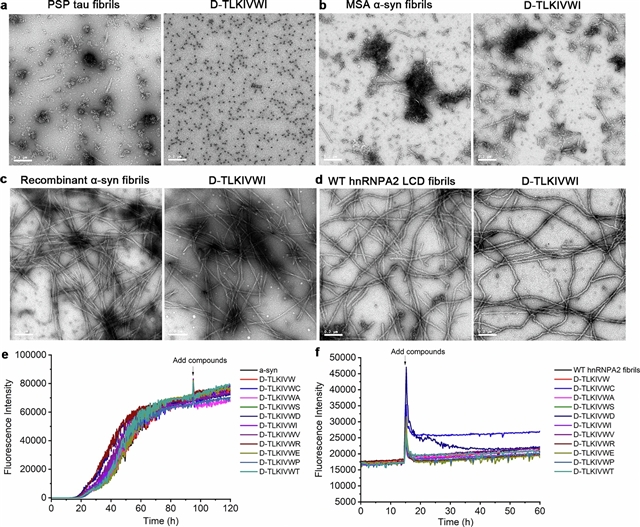
在此之前,课题组在体外发现,D-对构体肽(D-peptide) D-TLKIVWC可将AD患者尸体解剖的大脑中提取的超稳定的tau原纤维(以下简称AD-tau)分解成良性片段,除了环境热搅动外,不存在任何能量强度。为了考虑D肽介导的拆卸作为治疗AD的潜在途径,有必要了解拆卸作用的机制和能量强度。课题组发现D肽组装成淀粉样蛋白(“模拟淀粉样蛋白”)原纤维对于AD-tau的分解是必不可少的。这些模拟淀粉样蛋白原纤维具有右旋扭曲,但当与AD-tau复合模板时,它们被限制采用左旋扭曲。随着左旋到右旋的转变,松弛的模拟淀粉样蛋白释放的张力产生的扭矩足以破坏tau分子之间的局部氢键,并导致AD-tau的断裂。这种应变缓解机制似乎在淀粉样蛋白原纤维分解的其他例子中起作用,并可能为淀粉样蛋白疾病的一流治疗方法的发展提供信息。
据了解,减少tau蛋白的纤维聚集可能是阻止阿尔茨海默病(AD)进展的一种策略。
附:英文原文
Title: How short peptides disassemble tau fibrils in Alzheimer’s disease
Author: Hou, Ke, Ge, Peng, Sawaya, Michael R., Lutter, Liisa, Dolinsky, Joshua L., Yang, Yuan, Jiang, Yi Xiao, Boyer, David R., Cheng, Xinyi, Pi, Justin, Zhang, Jeffrey, Lu, Jiahui, Abskharon, Romany, Yang, Shixin, Yu, Zhiheng, Feigon, Juli, Eisenberg, David S.
Issue&Volume: 2025-07-09
Abstract: Reducing fibrous aggregates of the protein tau is a possible strategy for halting the progression of Alzheimer’s disease (AD)1. Previously, we found that in vitro, the D-enantiomeric peptide (D-peptide) D-TLKIVWC disassembles ultra-stable tau fibrils extracted from the autopsied brains of individuals with AD (hereafter, these tau fibrils are referred to as AD-tau) into benign segments, with no energy source other than ambient thermal agitation2. To consider D-peptide-mediated disassembly as a potential route to therapeutics for AD, it is essential to understand the mechanism and energy source of the disassembly action. Here, we show that the assembly of D-peptides into amyloid-like (‘mock-amyloid’) fibrils is essential for AD-tau disassembly. These mock-amyloid fibrils have a right-handed twist but are constrained to adopt a left-handed twist when templated in complex with AD-tau. The release of strain that accompanies the conversion of left-twisted to right-twisted, relaxed mock-amyloid produces a torque that is sufficient to break the local hydrogen bonding between tau molecules, and leads to the fragmentation of AD-tau. This strain-relief mechanism seems to operate in other examples of amyloid fibril disassembly, and could inform the development of first-in-class therapeutics for amyloid diseases.
DOI: 10.1038/s41586-025-09244-z
Source: https://www.nature.com/articles/s41586-025-09244-z
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
