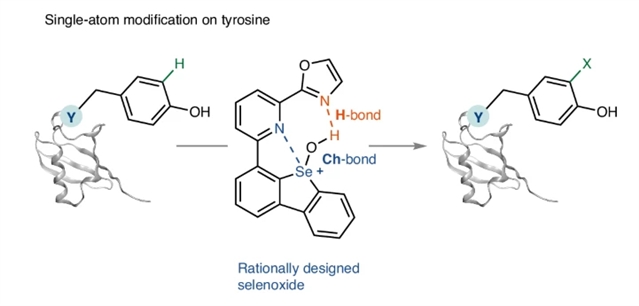
德国Kohlenforschung马克斯普朗克研究所Ritter, Tobias团队近日开发了一种用于酪氨酸残基单原子蛋白修饰的硒酸盐,通过耐水的硫和氢键来实现。相关论文发表在2025年6月4日出版的《自然-化学》杂志上。
翻译后修饰,如磷酸化和乙酰化,通常是微小的结构修饰,可以对蛋白质结构产生深远的影响,从而拓宽蛋白质功能。然而,直接研究这些效应往往遥不可及,因为没有通用的化学方法可以选择性地引入小的修饰;要么在蛋白质残基上选择性地安装一个大的、稳定的接头结构,要么引入一个小的取代基,由于使用反应性的、不加区分的分子,存在低选择性的风险。
研究组报告了酪氨酸残基的C-H官能化反应,以获得通过包括单原子置换在内的小结构变化修饰的肽和蛋白质。一种合理设计的硒氧化物引入了一种多功能硒关键蛋白,其特征是Ctyr-Se键,可用于在特定酪氨酸残基上进一步转化。这一进展的关键是耐水性、分子内硫属元素和硒氧化物试剂氢键的相互作用,这使得水溶液中酪氨酸残基的化学和位点选择性亲电芳香取代成为可能。
附:英文原文
Title: A selenoxide for single-atom protein modification of tyrosine residues enabled by water-resistant chalcogen and hydrogen bonding
Author: Lin, Songyun, Hirao, Marina, Hartmann, Philipp, Leutzsch, Markus, Sterling, Marie Sophie, Vetere, Alessandro, Klimmek, Sandra, Hinrichs, Heike, Mengeler, Johanna Marie, Lehmann, Johannes, Samsonowicz-Grski, Jan, Berger, Florian, Ritter, Tobias
Issue&Volume: 2025-06-04
Abstract: Post-translational modifications such as phosphorylation and acetylation are often minor structural modifications that can have profound effects on protein structure and thus broaden protein functions. Nevertheless, studying these effects directly is often out of reach because no general chemistry exists to introduce small modifications selectively; either a large, stable linker structure is selectively installed on protein residues, or a small substituent is introduced at the risk of low selectivity due to the use of reactive, indiscriminate molecules. Here we report a C–H functionalization reaction of tyrosine residues to access peptides and proteins modified by small structural changes including single-atom substitutions. A rationally designed selenoxide introduces a versatile selenonium linchpin featuring a Ctyr–Se bond that can be used for further transformations at specific tyrosine residues. Key to the advance is the interplay of water-resistant, intramolecular chalcogen and hydrogen bonding of the selenoxide reagent, which allows chemo- and site-selective electrophilic aromatic substitution of tyrosine residues in aqueous solutions.
DOI: 10.1038/s41557-025-01842-8
Source: https://www.nature.com/articles/s41557-025-01842-8
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
