近日,北京大学叶敏团队研究了植物类黄酮乙酰基转移酶糖受体选择性机制。相关论文发表在2025年6月3日出版的《美国化学会杂志》上。
黄酮类芹菜苷广泛分布于谷物、水果、蔬菜和草药中,对人类健康起着至关重要的作用。它们的简单高效合成一直是有机化学和生物合成领域的一个热门但具有挑战性的课题。然而,到目前为止,很少有关于芹菜酰基转移酶(ApiGTs)的报道。
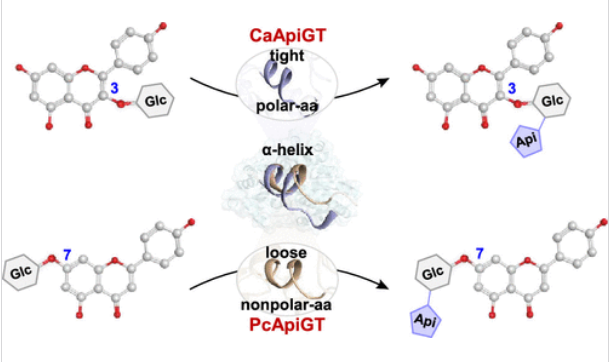
研究组报告了第一种能够催化鹰嘴豆(Cicer arietinum)中黄酮3-O-糖苷的2〃-O-芹菜基化的黄酮基转移酶(CaApiGT)。此外,从欧芹(Petroselinum crispum)中鉴定出PcApiGT,它催化黄酮类化合物7-/4′-O-糖苷的2〃-O-芹菜基化。为了剖析其不同糖受体选择性的机制,研究组获得了CaApiGT和PcApiGT的10个复杂晶体结构,分辨率范围为1.55至2.65Å,包括CaApiGT/UDP、CaApiGT/UDP/糖受体的6个三元结构、PcApiGT/UPD和PcApiGT/UDP的2个三元结构。
结构分析、理论计算和定点突变表明,黄酮3-O-糖苷和7-O-糖苷分别呈T形和流线型,符合CaApiGT和PcApiGT的活性囊。此外,这两种芹菜酰基转移酶的糖受体选择性是由一个关键的α-螺旋决定的。在CaApiGT中,这种α-螺旋包含多个极性氨基酸,特别是其末端的苏氨酸残基。使用这种α-螺旋基序作为标记,研究组进一步表征了来自豆科植物的四种与CaApiGT具有功能相似性的芹菜基转移酶。这项工作揭示了植物芹菜酰基转移酶的详细糖受体选择性机制,并为黄酮类芹菜苷的合成提供了有效的生物催化剂。
附:英文原文
Title: Insights into the Mechanisms of Sugar Acceptor Selectivity of Plant Flavonoid Apiosyltransferases
Author: Hao-Tian Wang, Zi-Long Wang, Nian-Hang Chen, Wei Huang, Jian-Lin Zou, Yun-Gang Tian, Guo Ye, Jian Huang, Ruibo Wu, Min Ye
Issue&Volume: June 3, 2025
Abstract: Flavonoid apiosides are widely distributed in cereals, fruits, vegetables, and medicinal herbs and play critical roles in human health. Their facile and efficient synthesis has been a hot but challenging topic in the fields of both organic chemistry and biosynthesis. However, very few apiosyltransferases (ApiGTs) have been reported thus far. Here, we report the first flavonoid apiosyltransferase (CaApiGT) capable of catalyzing the 2″-O-apiosylation of flavonoid 3-O-glycosides in chickpea (Cicer arietinum). Moreover, we identify PcApiGT from parsley (Petroselinum crispum), which catalyzes the 2″-O-apiosylation of flavonoid 7-/4′-O-glycosides. To dissect the mechanisms underlying their different sugar acceptor selectivity, we obtain 10 complex crystal structures of CaApiGT and PcApiGT with resolutions ranging from 1.55 to 2.65 , including CaApiGT/UDP, 6 ternary structures of CaApiGT/UDP/sugar acceptors, PcApiGT/UDP, and 2 ternary structures of PcApiGT/UDP/sugar acceptors. Structural analyses, theoretical calculations, and site-directed mutagenesis indicate that flavonoid 3-O-glycosides and 7-O-glycosides exhibit a T-shape and streamline shape, respectively, and fit the active pockets of CaApiGT and PcApiGT. Moreover, the sugar acceptor selectivity of these two apiosyltransferases is determined by a key α-helix. In CaApiGT, this α-helix contains multiple polar amino acids, particularly a threonine residue at its end. Using this α-helix motif as a marker, we further characterize four apiosyltransferases from Leguminosae plants that exhibit functional similarity to CaApiGT. This work unravels detailed sugar acceptor selectivity mechanisms of plant apiosyltransferases and provides efficient biocatalysts for the synthesis of flavonoid apiosides.
DOI: 10.1021/jacs.5c03771
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c03771
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
