瑞士苏黎世大学Raimund Dutzler研究团队宣布他们发现了TTYH2和APOE之间的相互作用促进了内体脂质转移。相关论文于2025年6月25日发表于国际顶尖学术期刊《自然》杂志上。
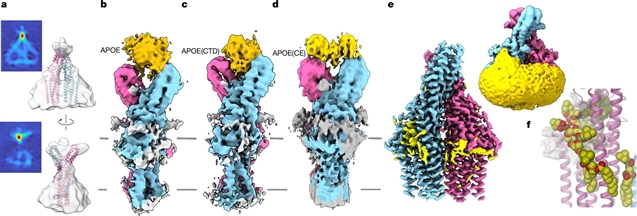
通过内源性蛋白的下拉,课题组确定APOE是人类TTYH2的相互作用伙伴。亚细胞分离和免疫细胞化学分析显示这两种蛋白在内体区室共定位。通过结合试验和结构研究表征APOE和TTYH2之间的特异性相互作用,使他们能够在TTYH2面向内体管腔的扩展区域中确定一个表位。与含APOE的脂蛋白颗粒的复合物结构显示出一种结合模式,将脂质放置在合适的位置,以促进其扩散到膜中。
此外,体外研究表明,TTYH2可以加速脂质转移。总的来说,他们的发现表明TTYH2在含有APOE的脂蛋白被内吞后的卸载中起作用。这些结果定义了一种新的蛋白质类,有助于从细胞膜中提取脂质并将其插入细胞膜。虽然它们无处不在,但这一过程可能与大脑特别相关,其中APOE参与了星形胶质细胞和神经元之间的脂质转移。
据了解,Tweety同源物(TTYHs)构成了一个真核生物膜蛋白家族,根据其结构特征,最近被提出参与可溶性载体和细胞膜之间的脂质转移。然而,在缺乏支持数据的情况下,这个函数是假设的。
附:英文原文
Title: Interactions between TTYH2 and APOE facilitate endosomal lipid transfer
Author: Sukalskaia, Anastasiia, Karner, Andreas, Pugnetti, Anna, Weber, Florian, Plochberger, Birgit, Dutzler, Raimund
Issue&Volume: 2025-06-25
Abstract: The Tweety homologues (TTYHs) constitute a family of eukaryotic membrane proteins that, on the basis of structural features, were recently proposed to contribute to lipid transfer between soluble carriers and cellular membranes1. However, in the absence of supporting data, this function was hypothetical. Here through pull-down of endogenous proteins, we identify APOE as the interaction partner of human TTYH2. Subcellular fractionation and immunocytochemistry assays showed that both proteins colocalize in endosomal compartments. Characterization of the specific interaction between APOE and TTYH2 through binding assays and structural studies enabled us to identify an epitope in an extended domain of TTYH2 that faces the endosomal lumen. Structures of complexes with APOE-containing lipoprotein particles revealed a binding mode that places lipids in a suitable position to facilitate their diffusion into the membrane. Moreover, in vitro studies revealed that lipid transfer is accelerated by TTYH2. Collectively, our findings indicate that TTYH2 has a role in the unloading of APOE-containing lipoproteins after they are endocytosed. These results define a new protein class that facilitates the extraction of lipids from and their insertion into cellular membranes. Although ubiquitous, this process could be of particular relevance in the brain, where APOE is involved in the transfer of lipids between astrocytes and neurons.
DOI: 10.1038/s41586-025-09200-x
Source: https://www.nature.com/articles/s41586-025-09200-x
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
