西南医科大学易东团队近日通过光诱导三组分碳酰化烯烃获得γ-氨基丁酸衍生物。该项研究成果发表在2025年6月23日出版的《中国化学》杂志上。
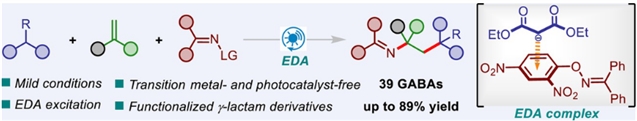
γ-氨基丁酸衍生物(GABAs)的显著生物活性促使人们探索绿色高效的合成方法来构建这些支架。研究组开发了一种无催化剂的光诱导策略,用于烯烃的氧化还原中性三组分碳酰化,实现了各种γ-氨基丁酸衍生物的高效和模块化组装。
机理研究表明,该反应是由去质子丙二酸酯和o -芳基肟之间的电子供体-受体络合物引发的。此外,所得产物可以通过酸性内酰胺化过程进一步转化为功能化γ-内酰胺衍生物。
附:英文原文
Title: Photoinduced Three-Component Carboimination 0f Alkenes to Access γ-Aminobutyric Acid Derivatives
Author: Lu Tan, Weicai Li, Shijing Tu, Xinyu Zhong, Xinhao Li, Xi Du, Zhijie Zhang, Siping Wei, Dong Yi
Issue&Volume: 2025-06-23
Abstract: The remarkable biological activities of γ-aminobutyric acid derivatives (GABAs) spurred the exploration of green and efficient synthetic methods to construct these scaffolds. Herein, we have developed a catalyst-free photoinduced strategy for the redox-neutral three-component carboimination of alkenes, enabling efficient and modular assembly of a wide range of γ-aminobutyric acid derivatives. Mechanistic studies indicate that this reaction is initiated with an electron donor-acceptor complex between deprotonated malonates and O-aryl oximes. Furthermore, the resulting products could be further converted to functionalized γ-lactam derivatives through an acidic lactamization process.
DOI: 10.1002/cjoc.70153
Source: https://onlinelibrary.wiley.com/doi/10.1002/cjoc.70153
Chinese Journal of Chemistry:《中国化学》,创刊于1983年。隶属于Wiley,最新IF:5.4
官方网址:https://onlinelibrary.wiley.com/journal/16147065
投稿链接:https://mc.manuscriptcentral.com/cjoc
