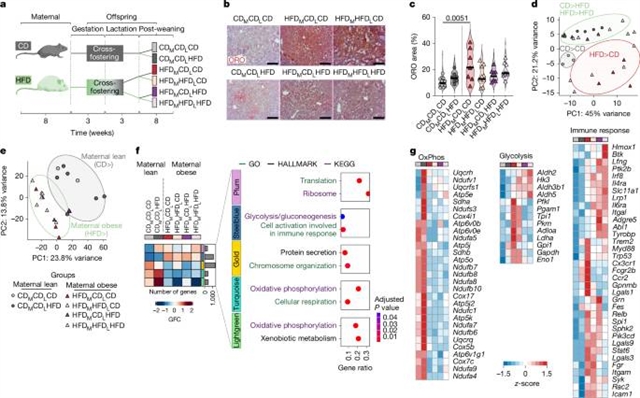
波恩大学Elvira Mass研究小组近日取得一项新成果。经过不懈努力,他们的最新研究揭示了母体肥胖导致的库普弗细胞编程引发脂肪肝。相关论文于2025年6月18日发表于国际顶尖学术期刊《自然》杂志上。
课题组人员利用母亲肥胖的母位模型来干扰妊娠期间的KC功能。课题组人员发现,暴露于母亲肥胖的后代会发展为脂肪肝疾病,这是由持续到成年的KCs异常发育程序驱动的。程序化KCs通过载脂蛋白分泌促进肝细胞脂质摄取。肥胖母亲所生的新生小鼠的KC消耗,随后补充幼稚单核细胞,挽救了脂肪肝疾病。
此外,妊娠期巨噬细胞中编码缺氧诱导因子-α (HIF1α)的基因的遗传消融可阻止KCs从氧化磷酸化到糖酵解的代谢程序,从而避免脂肪肝疾病的发展。这些结果确立了KC功能的发育扰动是成年期脂肪肝疾病的一个诱发因素,并将胎儿来源的巨噬细胞定位为健康和疾病发育起源概念中的关键代际信使。
据介绍,库普弗细胞(KCs)是在胚胎发生早期定植于肝脏的组织内巨噬细胞。在肝脏定植后,KCs迅速获得组织特异性转录特征,与发育中的肝脏一起成熟并适应其功能。在整个发育和成年过程中,KCs发挥着对肝脏和机体稳态至关重要的独特核心功能,包括支持胎儿红细胞生成、出生后红细胞循环和肝脏代谢。然而,发育过程中巨噬细胞核心功能的扰动是否会导致出生后阶段的巨噬细胞疾病尚不清楚。
附:英文原文
Title: Kupffer cell programming by maternal obesity triggers fatty liver disease
Author: Huang, Hao, Balzer, Nora R., Seep, Lea, Splichalova, Iva, Blank-Stein, Nelli, Viola, Maria Francesca, Franco Taveras, Eliana, Acil, Kerim, Fink, Diana, Petrovic, Franzisca, Makdissi, Nikola, Bayar, Seyhmus, Mauel, Katharina, Radwaniak, Carolin, Zurkovic, Jelena, Kayvanjoo, Amir H., Wunderling, Klaus, Jessen, Malin, Yaghmour, Mohamed H., Kenner, Lukas, Ulas, Thomas, Grein, Stephan, Schultze, Joachim L., Scott, Charlotte L., Guilliams, Martin, Liu, Zhaoyuan, Ginhoux, Florent, Beyer, Marc D., Thiele, Christoph, Meissner, Felix, Hasenauer, Jan, Wachten, Dagmar, Mass, Elvira
Issue&Volume: 2025-06-18
Abstract: Kupffer cells (KCs) are tissue-resident macrophages that colonize the liver early during embryogenesis1. Upon liver colonization, KCs rapidly acquire a tissue-specific transcriptional signature, mature alongside the developing liver and adapt to its functions1,2,3. Throughout development and adulthood, KCs perform distinct core functions that are essential for liver and organismal homeostasis, including supporting fetal erythropoiesis, postnatal erythrocyte recycling and liver metabolism4. However, whether perturbations of macrophage core functions during development contribute to or cause disease at postnatal stages is poorly understood. Here, we utilize a mouse model of maternal obesity to perturb KC functions during gestation. We show that offspring exposed to maternal obesity develop fatty liver disease, driven by aberrant developmental programming of KCs that persists into adulthood. Programmed KCs promote lipid uptake by hepatocytes through apolipoprotein secretion. KC depletion in neonate mice born to obese mothers, followed by replenishment with naive monocytes, rescues fatty liver disease. Furthermore, genetic ablation of the gene encoding hypoxia-inducible factor-α (HIF1α) in macrophages during gestation prevents the metabolic programming of KCs from oxidative phosphorylation to glycolysis, thereby averting the development of fatty liver disease. These results establish developmental perturbation of KC functions as a causal factor in fatty liver disease in adulthood and position fetal-derived macrophages as critical intergenerational messengers within the concept of developmental origins of health and diseases5.
DOI: 10.1038/s41586-025-09190-w
Source: https://www.nature.com/articles/s41586-025-09190-w
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
