近日,黑龙江大学郎凯团队研究了聚合物中线性共轭的可逆形成和控制。该研究于2025年6月19日发表在《自然-化学》杂志上。
有机半导体的出现为塑料电子领域奠定了基础。通过设计适当的共轭部分来控制π共轭是实现共轭材料所需半导体性能的最常见策略之一。尽管该领域取得了重大进展,但扩展共轭的可逆形成以在半导体和绝缘体之间原位切换大分子的性质仍然难以捉摸。
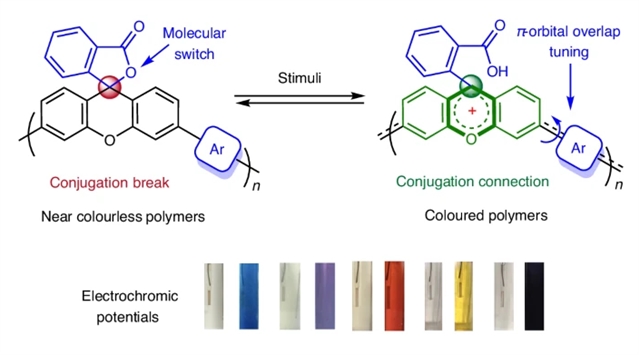
研究组公开了一种开发包含分子开关单元的聚合物结构的通用策略。这些单元能够响应酸碱或电子刺激,控制线性共轭的激活和失活。这是通过将非π共轭内酯官能化的黄原烯部分与传统的π共轭结构单元共聚来实现的。以2,6-二羟基萘为“电酸”,研究组探索了这些聚合物的电致变色特性,证明了非共轭无色聚合物有效地原位转化为共轭有色聚合物。
附:英文原文
Title: Reversible formation and control of linear conjugation in polymers
Author: Wu, Yanyun, Liu, Jiongjiang, Wang, Mengya, Wu, Xinzhou, Cai, Wanan, Niu, Haijun, Lang, Kai
Issue&Volume: 2025-06-19
Abstract: The emergence of organic semiconductors has laid the foundation for the field of plastic electronics. Controlling π-conjugation by designing proper conjugated moieties is one of the commonest strategies for achieving desired semiconducting properties in conjugated materials. Despite significant advancements in the field, the reversible formation of extended conjugation to in situ switch the nature of macromolecules between semiconductors and insulators remains elusive. Here we disclose a generic strategy for developing polymeric structures that incorporate molecular switch units. These units enable the controlled activation and deactivation of linear conjugation in response to acid–base or electronic stimuli. This is achieved by copolymerizing non-π-conjugated lactone-functionalized xanthene moieties with traditional π-conjugated building blocks. With 2,6-dihydroxynaphthalene as an ‘electro-acid’, the electrochromic-like properties of these polymers were explored, demonstrating an effective in situ conversion of the non-conjugated, colourless polymers into conjugated, coloured polymers.
DOI: 10.1038/s41557-025-01851-7
Source: https://www.nature.com/articles/s41557-025-01851-7
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
