近日,苏州大学谌宁团队研究了在富勒笼中稳定的镧碳三键。2025年6月19日,《自然-化学》杂志发表了这一成果。
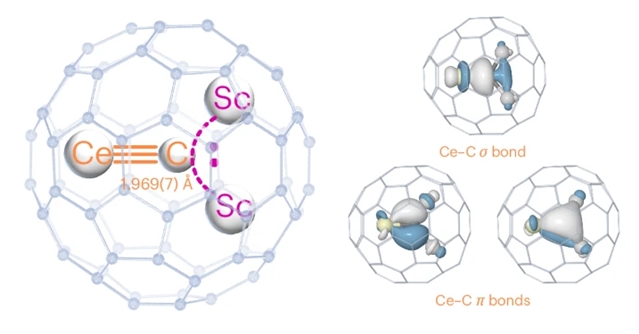
金属-配体多重键在配位和有机金属化学中无处不在。相比之下,镧系元素-碳多重键很难形成。含有镧系元素碳双键与末端甲基卡宾(=CH2)和镧系元素碳三键的配合物的分离仍然具有挑战性。研究组介绍了内嵌富勒烯笼内镧系元素-碳三键的合成。更具体地说,他们报道了一种封装在C80富勒烯笼内的碳化铈[Ce≡CSc2]团簇。
Ce≡CSc2的分子结构通过X射线晶体学、光谱分析和量子化学计算研究了Ce≡C三键的性质。该数据显示,Ce≡C距离非常短,为1.969(7)Å。化学键合分析表明,Ce≡C键的形成主要是由于与包封团簇内的钪相比,碳和铈之间的键合亲和力更强。富勒烯笼在稳定和保护这种具有Ce≡C三键的三金属碳化物簇中起着至关重要的作用。
附:英文原文
Title: A lanthanide–carbon triple bond stabilized within a fullerene cage
Author: Jiang, Hongjie, Zhao, Jing, Meng, Qingyu, Zhao, Xiao-Kun, Guo, Min, Hu, Han-Shi, Li, Jun, Chen, Ning
Issue&Volume: 2025-06-19
Abstract: Metal–ligand multiple bonds are ubiquitous in coordination and organometallic chemistry. In contrast, lanthanide–carbon multiple bonds are difficult to form. The isolation of complexes containing lanthanide–carbon double bonds with terminal methyl carbene (=CH2) and lanthanide–carbon triple bonds remains challenging. Here we present the synthesis of a lanthanide–carbon triple bond contained inside an endohedral fullerene cage. More specifically, we report a cerium–carbide [Ce≡CSc2] cluster encapsulated inside a C80 fullerene cage. The molecular structure of Ce≡CSc2@C80 and the nature of the Ce≡C triple bond are studied through X-ray crystallography, spectroscopic analyses and quantum chemical calculations. Our data reveal a very short Ce≡C distance of 1.969(7). Chemical bonding analysis suggests that the formation of the Ce≡C bond primarily arises from the stronger bonding affinity between carbon and cerium compared with scandium inside the encapsulated cluster. The fullerene cage plays a crucial role in stabilizing and protecting this trimetallic carbide cluster featuring a Ce≡C triple bond.
DOI: 10.1038/s41557-025-01856-2
Source: https://www.nature.com/articles/s41557-025-01856-2
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
