近日,南开大学鲁照永团队研究了1'-差向异构-septosones B和C的全合成及抗癌活性新化合物的发现。2025年6月18日出版的《中国化学》杂志发表了这项成果。
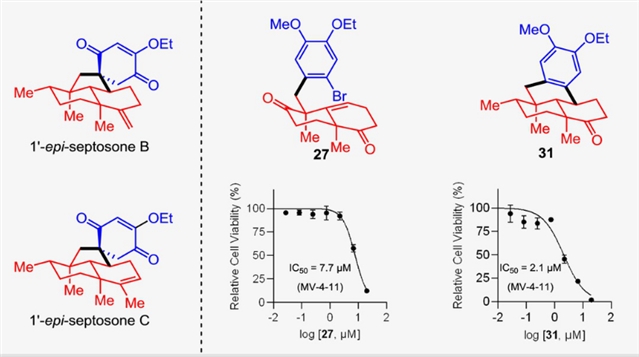
Septosones B和C是一对多环阿瓦烷型萜类酚,其拥有独特的螺[4.5]癸烷骨架,并表现出显著的生物活性。研究组报道了1'-差向异构-septosones B和C的全合成,其关键步骤是通过一个不寻常的立体专一性1,2-烷基迁移反应,将一个6/6稠合双烯酮叔醇转化为螺[4.5]烯二酮骨架。
更重要的是,通过对合成高级中间体的生物活性分析,发现两种新化合物对Hep G2、MV-4-11和MOLT-4细胞系表现出强效的抗癌活性,其IC50值低至2.1 μM。
附:英文原文
Title: Total Synthesis of 1'-epi-Septosones B and C and Discovery of Novel Compounds with Anti-Cancer Activity
Author: Qunlong Zhang, Jingyi Kang, Tiancheng Tan, Guanjun Dong, Jianwei Chen, Zhaoyong Lu
Issue&Volume: 2025-06-18
Abstract: Septosones B and C are a pair of polycyclic avarane-type meroterpenoids which possess a distinctive spiro[4.5]decane backbone and exhibit promising biological activity. We report here the total synthesis of 1'-epi-septosones B and C through an unusual stereospecific 1,2-alkyl migration of a 6/6-fused dienone tertiary alcohol to a spiro[4.5]enedione scaffold. More importantly, two new compounds were found to exhibit potent anti-cancer activity against Hep G2, MV-4-11, and MOLT-4 cell lines with IC50 values as low as 2.1 μM through bioactivity profiling of the synthetic advanced intermediates.
DOI: 10.1002/cjoc.70121
Source: https://onlinelibrary.wiley.com/doi/10.1002/cjoc.70121
Chinese Journal of Chemistry:《中国化学》,创刊于1983年。隶属于Wiley,最新IF:5.4
官方网址:https://onlinelibrary.wiley.com/journal/16147065
投稿链接:https://mc.manuscriptcentral.com/cjoc
