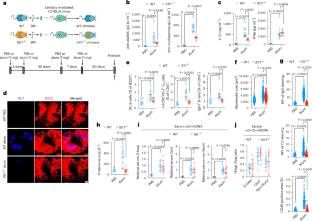
基因调控与信号转导实验室Michael Karin课题组近日取得一项新成果。经过不懈努力,他们的研究报道了线粒体DNA氧化通过使浆细胞样树突状细胞诱导TFH分化来传播自身免疫。2025年6月17日出版的《自然—免疫学》杂志发表了这项成果。
本研究表明,由一种典型的NLRP3炎性小体激活剂触发的Ox-mtDNA持续释放可诱导小鼠产生自身抗体和肾小球肾炎。类似的自身免疫反应,依赖于浆细胞样树突状细胞(pDCs)和滤泡辅助性T细胞(TFH),可由体外生成的Ox-mtDNA引起,而非氧化mtDNA则不能。尽管两种mtDNA形式都被pDCs内化并诱导干扰素-α,但只有Ox-mtDNA刺激自分泌白细胞介素(IL)-1β信号,诱导共刺激分子和IL-21,从而使motheme和人pDCs诱导功能性TFH分化,支持自身抗体的产生。这些发现强调了pDCs产生的IL-1β在自身抗体产生中的作用,并强调了Ox-mtDNA是一个重要的自身免疫触发因素,提示潜在的治疗机会。
据悉,应激诱导的氧化线粒体DNA (Ox-mtDNA)片段进入细胞质,激活NLRP3炎性体和caspase-1,并使气真皮蛋白D介导的mtDNA循环释放。在老年人和患有代谢或自身免疫性疾病的患者中检测到循环mtDNA含量升高,可能被氧化。
附:英文原文
Title: Mitochondrial DNA oxidation propagates autoimmunity by enabling plasmacytoid dendritic cells to induce TFH differentiation
Author: Xian, Hongxu, Watari, Kosuke, Ohira, Masafumi, Brito, Jonathan S., He, Peng, Onyuru, Janset, Zuniga, Elina I., Hoffman, Hal M., Karin, Michael
Issue&Volume: 2025-06-17
Abstract: Stress-induced oxidized mitochondrial DNA (Ox-mtDNA) fragments enter the cytoplasm, activating the NLRP3 inflammasome and caspase-1 and enabling gasdermin-D-mediated circulatory release of mtDNA. Elevated amounts of circulating mtDNA, presumably oxidized, have been detected in older individuals and patients with metabolic or autoimmune disorders. Here we show that sustained Ox-mtDNA release, triggered by a prototypical NLRP3 inflammasome activator, induces autoantibody production and glomerulonephritis in mice. Similar autoimmune responses, dependent on plasmacytoid dendritic cells (pDCs) and follicular helper T (TFH) cells, are elicited by in vitro-generated Ox-mtDNA, but not by non-oxidized mtDNA. Although both mtDNA forms are internalized by pDCs and induce interferon-α, only Ox-mtDNA stimulates autocrine interleukin (IL)-1β signaling that induces co-stimulatory molecules and IL-21, which enable mouse and human pDCs to induce functional TFH differentiation, supportive of autoantibody production. These findings underscore the role of pDC-generated IL-1β in autoantibody production and highlight Ox-mtDNA as an important autoimmune trigger, suggesting potential therapeutic opportunities.
DOI: 10.1038/s41590-025-02179-7
Source: https://www.nature.com/articles/s41590-025-02179-7
Nature Immunology:《自然—免疫学》,创刊于2000年。隶属于施普林格·自然出版集团,最新IF:31.25
官方网址:https://www.nature.com/ni/
投稿链接:https://mts-ni.nature.com/cgi-bin/main.plex
