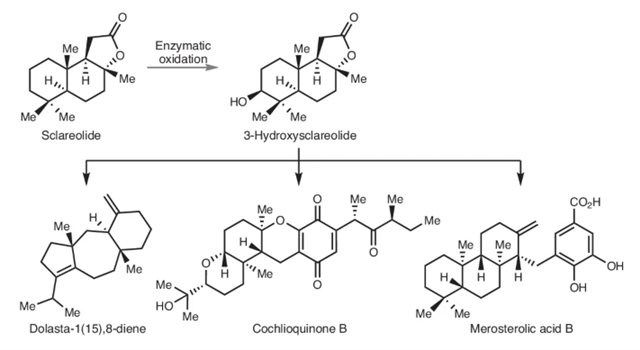
上海交通大学李健团队近日通过酶促驱动的非生物骨架重构实现多样化萜类结构体系合成。该项研究成果发表在2025年6月16日出版的《Nature Chemistry》上。
由于天然产物的结构复杂性,它们的靶向合成通常需要为特定靶点量身定制个性化路线的设计。因此,当考虑不同骨架连接的目标时,需要重新设计路线。
研究组报告了一种通用的合成策略,该策略与这种传统观点背道而驰,允许从倍半萜内酯-香紫苏内酯作为起始原料获得一系列具有不同骨架的萜类化合物。通过将生物催化引入的羟基视为可利用基团而非结构终点,设计出若干非生物骨架重排反应,从而显著分化了源自硬尾醇内酯的原始泪柏烷环系结构。基于此策略,成功实现了四种萜类天然产物——霉甾醇酸B、旋喹酮B、(+)-胡萝卜烯以及海兔-1(15),8-二烯的全合成。
附:英文原文
Title: Synthesis of diverse terpenoid frameworks via enzyme-enabled abiotic scaffold hop
Author: Deng, Heping, Yang, Junhong, Li, Fuzhuo, Li, Jian, Renata, Hans
Issue&Volume: 2025-06-16
Abstract: Due to the structural complexity of natural products, their target-oriented syntheses usually require the design of individualized routes that are tailor-made for the specific targets. As such, route redesign is needed when targets of different skeletal connectivities are considered. Here we report a versatile synthetic strategy that runs counter to this conventional wisdom and allows access to a range of terpenoids with distinct skeletal frameworks from the sesquiterpene lactone sclareolide as the starting material. By viewing a biocatalytically installed alcohol as an exploitable motif rather than a structural endpoint, a number of abiotic skeletal rearrangements were designed, resulting in substantial structural divergence from the original drimane ring system of sclareolide. Using this approach, the syntheses of four terpenoid natural products, namely, merosterolic acid B, cochlioquinone B, (+)-daucene and dolasta-1(15),8-diene, were achieved.
DOI: 10.1038/s41557-025-01852-6
Source: https://www.nature.com/articles/s41557-025-01852-6
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
