南开大学王晓晨团队实现了通过选择性地在C-C键中插入炔在胺中的模烷基生长。相关论文发表在2025年6月12日出版的《自然-化学》杂志上。
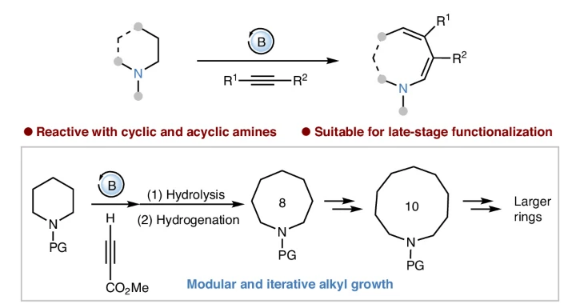
胺的位点特异性修饰一直是有机合成中备受追捧的目标。尽管碳-氢(C-H)键官能化方法发展迅速,但胺的碳-碳(C-C)键功能化的有效策略仍然难以实现。研究组报告了一种硼烷催化的方法,用于将炔烃选择性插入胺的烷基C-C键,导致环胺的环膨胀和无环胺的链伸长。
这种方法从胺中C-H键的断裂开始,然后在与炔烃反应后过渡到C-C键的官能化。该方法对缺乏活化或离去基团的胺有效,适用于通过C-C键修饰对药物进行后期功能化。此外,通过将该反应与水解和氢化步骤耦合,实现了连续的炔烃插入,使胺的模块化和迭代烷基生长成为可能。
附:英文原文
Title: Modular alkyl growth in amines via the selective insertion of alkynes into C–C bonds
Author: Zhou, Xin-Yue, Liu, Lu, Lyu, Hairong, Wang, Xiao-Chen
Issue&Volume: 2025-06-12
Abstract: The site-specific modification of amines has been a highly sought-after objective in organic synthesis. Despite the rapid advancement of carbon–hydrogen (C–H) bond functionalization methods, effective strategies for carbon–carbon (C–C) bond functionalization of amines remain elusive. Here we report a borane-catalysed method for the selective insertion of alkynes into alkyl C–C bonds of amines, resulting in the ring expansion of cyclic amines and chain elongation of acyclic amines. This approach begins with the cleavage of C–H bonds in amines, then transitioning to C–C bond functionalization upon reaction with alkynes. This method is effective with amines lacking an activating or leaving group and is suitable for late-stage functionalization of pharmaceuticals through C–C bond modification. Furthermore, by coupling this reaction with hydrolysis and hydrogenation steps, successive alkyne insertions are achieved, enabling modular and iterative alkyl growth of amines.
DOI: 10.1038/s41557-025-01849-1
Source: https://www.nature.com/articles/s41557-025-01849-1
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
