近日,上海大学龚和贵团队研究了镍催化不对称还原交叉偶联制备手性α-芳基酮和醛的统一方法。这一研究成果于2025年5月8日发表在《美国化学会志》上。
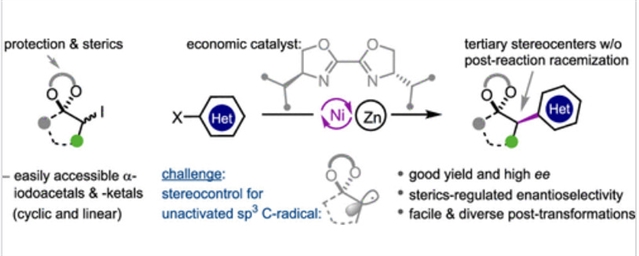
研究组公开了一种高度对映选择性的方案,用于轻松制备受保护的叔α-芳基酮和醛,该方案通过手性Ni/Biox催化的易于获得的α-碘缩醛和-酮与(杂)芳基卤化物的还原偶联来实现。该方法的通用性通过其在广泛的(杂环)环状和无环羰基支架上的优异性能得到了证明。该反应被认为是通过未活化的缩醛/缩酮α-碳自由基进行的,克服了与缩醛/缩甲醛部分的辅助空间体积以及缺乏稳定和指导因素相关的挑战。
实验和密度泛函理论(DFT)研究揭示了一种合理的自由基链机制,并阐明了观察到的对映选择性。该方法避免了在无保护的叔α-芳基酮和醛中经常遇到的反应后差向异构化问题。因此,利用富含地球的镍催化剂,它为基于叔α-芳基酮和醛快速合成各种重要的天然产物和药物分子带来了巨大的希望。
附:英文原文
Title: A Unified Approach to Chiral α-Aryl Ketones and Aldehydes via Ni-Catalyzed Asymmetric Reductive Cross-Coupling
Author: Canbin Qiu, Lin Liu, Keyang Zhang, Shanshan Du, Yunrong Chen, Xiaotai Wang, Hegui Gong
Issue&Volume: May 8, 2025
Abstract: We disclose a highly enantioselective protocol for the facile preparation of protected tertiary α-aryl ketones and aldehydes, enabled by chiral Ni/Biox-catalyzed reductive coupling of readily accessible α-iodoacetals and -ketals with (hetero)aryl halides. The generality of the method is demonstrated by its excellent performance across a broad range of (hetero)cyclic and acyclic carbonyl scaffolds. The reaction is thought to proceed through unactivated acetal/ketal α-carbon radicals, overcoming challenges related to the ancillary steric bulkiness of the acetal/ketal moieties and the lack of stabilizing and directing factors. Experimental and density functional theory (DFT) studies reveal a plausible radical chain mechanism and elucidate the observed enantioselectivity. This method avoids the postreaction epimerization issue often encountered in unprotected tertiary α-aryl ketones and aldehydes. Thus, it holds great promise for the rapid synthesis of a wide array of important natural products and drug molecules based on tertiary α-aryl ketones and aldehydes by leveraging earth-abundant nickel catalysts.
DOI: 10.1021/jacs.5c03418
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c03418
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
