近日,英国曼彻斯特大学教授Green, Anthony P.团队研究了可见光驱动的高效选择性能量转移光酶。该研究于2025年5月6日发表在《自然-化学》杂志上。
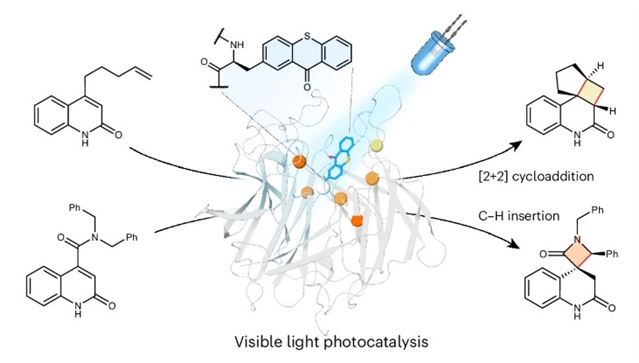
含有二苯甲酮三重态敏化剂的[2+2]环化酶的开发突显了工程酶作为立体控制能量转移光催化平台的潜力。然而,二苯甲酮的次优光物理特性需要使用紫外光,限制了光化学效率,并限制了可获得的化学物质的范围。研究组设计了一种正交的詹氏甲烷球菌酪氨酰-tRNA合成酶/tRNA组合,用于将噻吨酮三重敏化剂编码成蛋白质,这可以有效地利用可见光来驱动光化学转化。
最初,研究组开发了一种对映选择性[2+2]环化酶,其效率比之前开发的光酶(kcat = 13 s−1,>1300次失误)高出几个数量级。为了证明含噻吨酮的酶可以实现更具挑战性的光化学转化,他们开发了第二种耐氧酶,可以引导激发的喹诺酮底物的选择性C-H插入,以高选择性(99%e.e.,22:1 d.r.)提供螺环β-内酰胺。这种光酶还抑制了小分子敏化剂观察到的竞争性底物分解途径,突显了工程酶控制激发态中间体命运的能力。
附:英文原文
Title: Efficient and selective energy transfer photoenzymes powered by visible light
Author: Crawshaw, Rebecca, Smithson, Ross, Hofer, Johannes, Hardy, Florence J., Roberts, George W., Trimble, Jonathan S., Kohn, Anna R., Levy, Colin W., Drost, Deborah A., Merten, Christian, Heyes, Derren J., Obexer, Richard, Bach, Thorsten, Green, Anthony P.
Issue&Volume: 2025-05-06
Abstract: The development of [2+2] cyclases containing benzophenone triplet sensitizers highlights the potential of engineered enzymes as a platform for stereocontrolled energy transfer photocatalysis. However, the suboptimal photophysical features of benzophenone necessitates the use of ultraviolet light, limits photochemical efficiency and restricts the range of chemistries accessible. Here we engineer an orthogonal Methanococcus jannaschii tyrosyl-tRNA synthetase/tRNA pair for encoding thioxanthone triplet sensitizers into proteins, which can efficiently harness visible light to drive photochemical conversions. Initially, we developed an enantioselective [2+2] cyclase that is orders of magnitude more efficient than our previously developed photoenzymes (kcat=13s1, >1,300 turnovers). To demonstrate that thioxanthone-containing enzymes can enable more challenging photochemical conversions, we developed a second oxygen-tolerant enzyme that can steer selective C–H insertions of excited quinolone substrates to afford spirocyclic β-lactams with high selectivity (99% e.e., 22:1 d.r.). This photoenzyme also suppresses a competing substrate decomposition pathway observed with small-molecule sensitizers, underscoring the ability of engineered enzymes to control the fate of excited-state intermediates.
DOI: 10.1038/s41557-025-01820-0
Source: https://www.nature.com/articles/s41557-025-01820-0
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
